Environmental Tobacco Smoke Alters Metabolic Systems in Adult Rats
- PMID: 27788581
- PMCID: PMC6707370
- DOI: 10.1021/acs.chemrestox.6b00187
Environmental Tobacco Smoke Alters Metabolic Systems in Adult Rats
Abstract
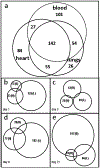
Human exposure to environmental tobacco smoke (ETS) is associated with an increased incidence of pulmonary and cardiovascular disease and possibly lung cancer. Metabolomics can reveal changes in metabolic networks in organisms under different physio-pathological conditions. Our objective was to identify spatial and temporal metabolic alterations with acute and repeated subchronic ETS exposure to understand mechanisms by which ETS exposure may cause adverse physiological and structural changes in the pulmonary and cardiovascular systems. Established and validated metabolomics assays of the lungs, hearts. and blood of young adult male rats following 1, 3, 8, and 21 days of exposure to ETS along with day-matched sham control rats (n = 8) were performed using gas chromatography time-of-flight mass spectrometry, BinBase database processing, multivariate statistical modeling, and MetaMapp biochemical mapping. A total of 489 metabolites were measured in the lung, heart, and blood, of which 142 metabolites were identified using a standardized metabolite annotation pipeline. Acute and repeated subchronic exposure to ETS was associated with significant metabolic changes in the lung related to energy metabolism, defense against reactive oxygen species, substrate uptake and transport, nucleotide metabolism, and substrates for structural components of collagen and membrane lipids. Metabolic changes were least prevalent in heart tissues but abundant in blood under repeated subchronic ETS exposure. Our analyses revealed that ETS causes alterations in metabolic networks, especially those associated with lung structure and function and found as systemic signals in the blood. The metabolic changes suggest that ETS exposure may adversely affects the mitochondrial respiratory chain, lung elasticity, membrane integrity, redox states, cell cycle, and normal metabolic and physiological functions of the lungs, even after subchronic ETS exposure.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
-
- Oberg M, Jaakkola MS, Woodward A, Peruga A, and Pruss-Ustun A (2011) Worldwide burden of disease from exposure to second-hand smoke: a retrospective analysis of data from 192 countries. Lancet 377, 139–146. - PubMed
-
- U.S. Surgeon General (2006) The Health Consequences of Involuntary Exposure to Tobacco Smoke: A Report of the Surgeon General, US Department of Health and Human Services, Centers for Disease Control and Prevention, Coordinating Center for Health Promotion, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health, Atlanta, GA, http://www.surgeongeneral.gov/library/secondhandsmoke/.
-
- Chilmonczyk BA, Salmun LM, Megathlin KN, Neveux LM, Palomaki GE, Knight GJ, Pulkkinen AJ, and Haddow JE (1993) Association between exposure to environmental tobacco smoke and exacerbations of asthma in children. N. Engl. J. Med 328, 1665–1669. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

