MOG-IgG in NMO and related disorders: a multicenter study of 50 patients. Part 1: Frequency, syndrome specificity, influence of disease activity, long-term course, association with AQP4-IgG, and origin
- PMID: 27788675
- PMCID: PMC5084340
- DOI: 10.1186/s12974-016-0717-1
MOG-IgG in NMO and related disorders: a multicenter study of 50 patients. Part 1: Frequency, syndrome specificity, influence of disease activity, long-term course, association with AQP4-IgG, and origin
Abstract
Background: Antibodies to myelin oligodendrocyte glycoprotein (MOG-IgG) have been suggested to play a role in a subset of patients with neuromyelitis optica and related disorders.
Objective: To assess (i) the frequency of MOG-IgG in a large and predominantly Caucasian cohort of patients with optic neuritis (ON) and/or myelitis; (ii) the frequency of MOG-IgG among AQP4-IgG-positive patients and vice versa; (iii) the origin and frequency of MOG-IgG in the cerebrospinal fluid (CSF); (iv) the presence of MOG-IgG at disease onset; and (v) the influence of disease activity and treatment status on MOG-IgG titers.
Methods: 614 serum samples from patients with ON and/or myelitis and from controls, including 92 follow-up samples from 55 subjects, and 18 CSF samples were tested for MOG-IgG using a live cell-based assay (CBA) employing full-length human MOG-transfected HEK293A cells.
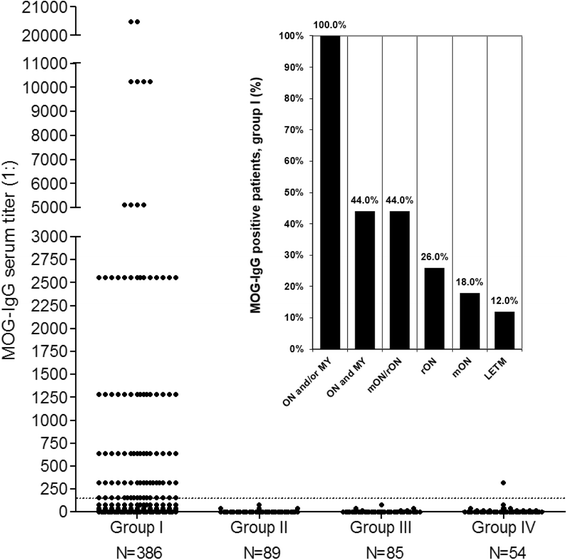
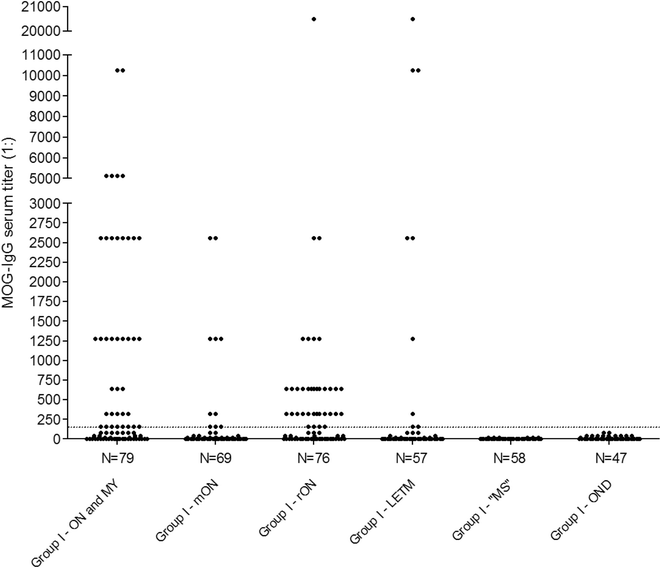
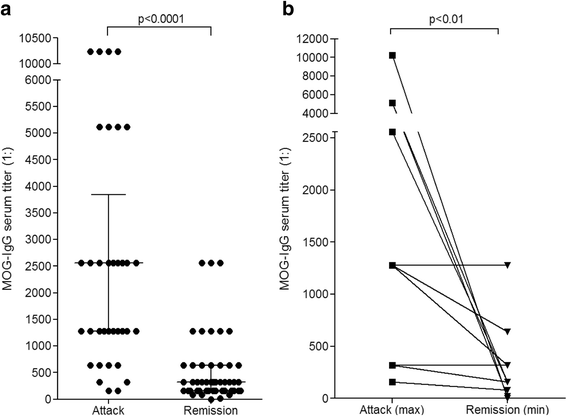
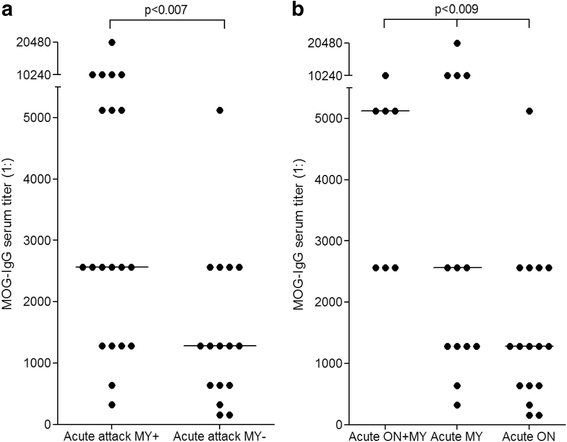
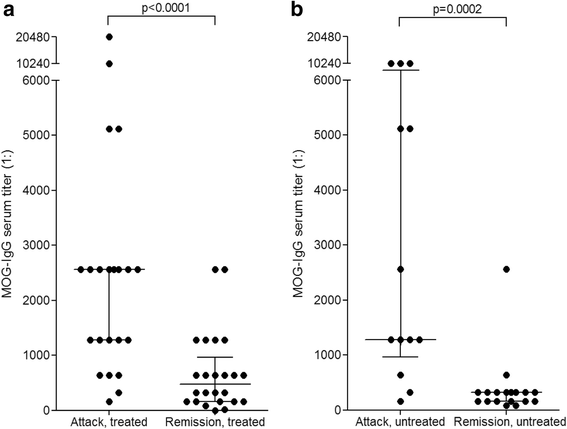
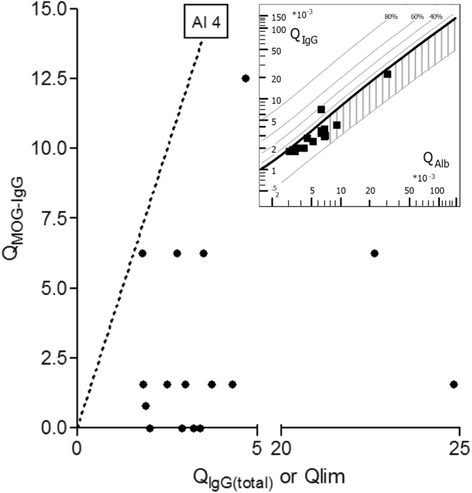
Results: MOG-IgG was detected in 95 sera from 50 patients with ON and/or myelitis, including 22/54 (40.7 %) patients with a history of both ON and myelitis, 22/103 (21.4 %) with a history of ON but no myelitis and 6/45 (13.3 %) with a history of longitudinally extensive transverse myelitis but no ON, and in 1 control patient with encephalitis and a connective tissue disorder, all of whom were negative for AQP4-IgG. MOG-IgG was absent in 221 further controls, including 83 patients with AQP4-IgG-seropositive neuromyelitis optica spectrum disorders and 85 with multiple sclerosis (MS). MOG-IgG was found in 12/18 (67 %) CSF samples from MOG-IgG-seropositive patients; the MOG-IgG-specific antibody index was negative in all cases, indicating a predominantly peripheral origin of CSF MOG-IgG. Serum and CSF MOG-IgG belonged to the complement-activating IgG1 subclass. MOG-IgG was present already at disease onset. The antibodies remained detectable in 40/45 (89 %) follow-up samples obtained over a median period of 16.5 months (range 0-123). Serum titers were higher during attacks than during remission (p < 0.0001), highest during attacks of simultaneous myelitis and ON, lowest during acute isolated ON, and declined following treatment.
Conclusions: To date, this is the largest cohort studied for IgG to human full-length MOG by means of an up-to-date CBA. MOG-IgG is present in a substantial subset of patients with ON and/or myelitis, but not in classical MS. Co-existence of MOG-IgG and AQP4-IgG is highly uncommon. CSF MOG-IgG is of extrathecal origin. Serum MOG-IgG is present already at disease onset and remains detectable in the long-term course. Serum titers depend on disease activity and treatment status.
Keywords: Antibody index; Aquaporin-4 antibodies (AQP4-IgG); Autoantibodies; Cell-based assays; Cerebrospinal fluid; Devic’s syndrome; Longitudinally extensive transverse myelitis (LETM); Multiple sclerosis; Myelin oligodendrocyte glycoprotein antibodies (MOG-IgG); Neuromyelitis optica antibodies (NMO-IgG); Neuromyelitis optica spectrum disorders (NMOSD); Neuromyelitis optica (NMO); Optic neuritis; Transverse Myelitis.
Figures


References
-
- Jarius S, Paul F, Franciotta D, Waters P, Zipp F, Hohlfeld R, Vincent A, Wildemann B. Mechanisms of Disease: Aquaporin-4 antibodies in neuromyelitis optica. Nat Clin Pract Neurol. 2008;4:202–14. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

