Visually-guided gait training in paretic patients during the first rehabilitation phase: study protocol for a randomized controlled trial
- PMID: 27788679
- PMCID: PMC5081976
- DOI: 10.1186/s13063-016-1630-8
Visually-guided gait training in paretic patients during the first rehabilitation phase: study protocol for a randomized controlled trial
Abstract
Background: After a lesion to the central nervous system, many patients suffer from reduced walking capability. In the first rehabilitation phase, repeated walking exercises facilitate muscular strength and stimulate brain plasticity and motor relearning. However, marked limping, an unsteady gait, and poor management of obstacle clearance may persist, which increases a patient's risk of falling. Gait training with augmented reality has been recommended to improve gait coordination. The objective of this study is to test whether a gait rehabilitation program using augmented reality is superior to a conventional treadmill training program of equivalent intensity.
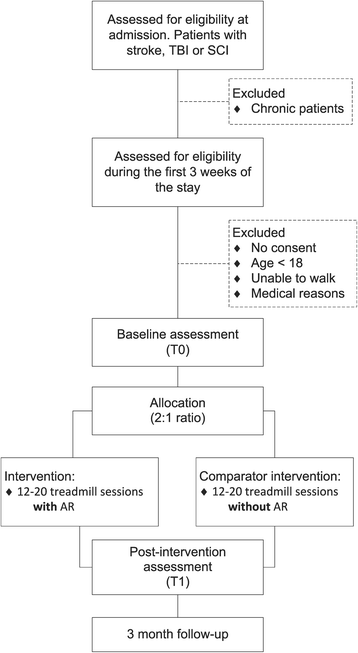
Methods/design: The GASPAR trial (Gait Adaptation for Stroke Patients with Augmented Reality) is a pragmatic, parallel-arm, single-center, nonblind, superiority randomized control trial in neurorehabilitation. The setting is a rehabilitation clinic in Switzerland. The planned number of participants is 70-100. The intervention uses instrumented treadmills equipped with projectors that display shapes on the walking surface. The principle is that patients must adapt their gait to the image that unfolds in front of them. Specific exercises for gait symmetry, coordination enhancement, and gait agility are provided. The program includes twenty 30-min sessions spanning 4 weeks. The comparator group receives standard treadmill training of a similar frequency and intensity. The main outcome to be measured in the trial is walking speed, which is assessed with the 2-min Walk Test. Moreover, gait parameters are recorded during the gait training sessions. Other outcomes are balance control (Berg Balance Scale) and the fear of falling (Falls Efficacy Scale). The statistical analyses will compare the baseline assessment for each participant (before the intervention) with a post-intervention assessment (taken a few days after the end of the program). Furthermore, a follow-up assessment will take place 3 months after discharge.
Discussion: The study results will provide new knowledge about recovery in neurological patients and will contribute to the design of better rehabilitation programs to accompany this process. The findings will also help health care funders to decide whether treadmills equipped with augmented reality capabilities are a worthwhile investment.
Trial registration: ClinicalTrials.gov ID: NCT02808078 , registered on 16 June 2016.
Keywords: Augmented reality; Randomized controlled trial; Rehabilitation; Spinal cord injury; Stroke; Traumatic brain injury.
Figures
References
-
- Meyer K, Simmet A, Arnold M, Mattle H, Nedeltchev K. Stroke events and case fatalities in Switzerland based on hospital statistics and cause of death statistics. Swiss Med Wkly. 2009;139:65–9. - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical