Local consolidative therapy versus maintenance therapy or observation for patients with oligometastatic non-small-cell lung cancer without progression after first-line systemic therapy: a multicentre, randomised, controlled, phase 2 study
- PMID: 27789196
- PMCID: PMC5143183
- DOI: 10.1016/S1470-2045(16)30532-0
Local consolidative therapy versus maintenance therapy or observation for patients with oligometastatic non-small-cell lung cancer without progression after first-line systemic therapy: a multicentre, randomised, controlled, phase 2 study
Abstract
Background: Evidence from retrospective studies suggests that disease progression after first-line chemotherapy for metastatic non-small-cell lung cancer (NSCLC) occurs most often at sites of disease known to exist at baseline. However, the potential effect of aggressive local consolidative therapy for patients with oligometastatic NSCLC is unknown. We aimed to assess the effect of local consolidative therapy on progression-free survival.
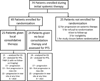
Methods: In this multicentre, randomised, controlled, phase 2 study, eligible patients from three hospitals had histological confirmation of stage IV NSCLC, three or fewer metastatic disease lesions after first-line systemic therapy, an Eastern Cooperative Oncology Group performance status score of 2 or less, had received standard first-line systemic therapy, and had no disease progression before randomisation. First-line therapy was four or more cycles of platinum doublet therapy or 3 or more months of EGFR or ALK inhibitors for patients with EGFR mutations or ALK rearrangements, respectively. Patients were randomly assigned (1:1) to either local consolidative therapy ([chemo]radiotherapy or resection of all lesions) with or without subsequent maintenance treatment or to maintenance treatment alone, which could be observation only. Maintenance treatment was recommended based on a list of approved regimens, and observation was defined as close surveillance without cytotoxic treatment. Randomisation was not masked and was balanced dynamically on five factors: number of metastases, response to initial therapy, CNS metastases, intrathoracic nodal status, and EGFR and ALK status. The primary endpoint was progression-free survival analysed in all patients who were treated and had at least one post-baseline imaging assessment. The study is ongoing but not recruiting participants. This study is registered with ClinicalTrials.gov, number NCT01725165.
Findings: Between Nov 28, 2012, and Jan 19, 2016, 74 patients were enrolled either during or at the completion of first-line systemic therapy. The study was terminated early after randomisation of 49 patients (25 in the local consolidative therapy group and 24 in the maintenance treatment group) as part of the annual analyses done by the Data Safety Monitoring Committee of all randomised trials at MD Anderson Cancer Center, and before a planned interim analysis of 44 events. At a median follow-up time for all randomised patients of 12·39 months (IQR 5·52-20·30), the median progression-free survival in the local consolidative therapy group was 11·9 months (90% CI 5·7-20·9) versus 3·9 months (2·3-6·6) in the maintenance treatment group (hazard ratio 0·35 [90% CI 0·18-0·66], log-rank p=0·0054). Adverse events were similar between groups, with no grade 4 adverse events or deaths due to treatment. Grade 3 adverse events in the maintenance therapy group were fatigue (n=1) and anaemia (n=1) and in the local consolidative therapy group were oesophagitis (n=2), anaemia (n=1), pneumothorax (n=1), and abdominal pain (n=1, unlikely related).
Interpretation: Local consolidative therapy with or without maintenance therapy for patients with three or fewer metastases from NSCLC that did not progress after initial systemic therapy improved progression-free survival compared with maintenance therapy alone. These findings suggest that aggressive local therapy should be further explored in phase 3 trials as a standard treatment option in this clinical scenario.
Funding: MD Anderson Lung Cancer Priority Fund, MD Anderson Cancer Center Moon Shot Initiative, and Cancer Center Support (Core), National Cancer Institute, National Institutes of Health.
Copyright © 2016 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of Interest: The other authors declared no conflicts of interest.
Figures
Comment in
-
Radical treatment of synchronous oligometastases from NSCLC.Lancet Oncol. 2016 Dec;17(12):1625-1626. doi: 10.1016/S1470-2045(16)30533-2. Epub 2016 Oct 24. Lancet Oncol. 2016. PMID: 27789197 No abstract available.
-
Local consolidative therapy may be beneficial in patients with oligometastatic non-small cell lung cancer.CA Cancer J Clin. 2017 Mar;67(2):89-90. doi: 10.3322/caac.21363. Epub 2017 Jan 17. CA Cancer J Clin. 2017. PMID: 28094844 No abstract available.
-
Consolidative local therapy in oligometastatic patients.Lancet Oncol. 2017 Feb;18(2):e60. doi: 10.1016/S1470-2045(17)30014-1. Lancet Oncol. 2017. PMID: 28214414 No abstract available.
-
Consolidative local therapy in oligometastatic patients.Lancet Oncol. 2017 Feb;18(2):e61. doi: 10.1016/S1470-2045(17)30013-X. Lancet Oncol. 2017. PMID: 28214415 No abstract available.
-
Consolidative local therapy in oligometastatic patients - Authors' reply.Lancet Oncol. 2017 Feb;18(2):e62. doi: 10.1016/S1470-2045(17)30028-1. Lancet Oncol. 2017. PMID: 28214416 No abstract available.
-
[Consolidative local therapy in oligometastatic NSCLC without progression after first-line chemotherapy].Strahlenther Onkol. 2017 Apr;193(4):341-343. doi: 10.1007/s00066-017-1113-1. Strahlenther Onkol. 2017. PMID: 28236204 German. No abstract available.
-
Is there a benefit to locally consolidative therapy for oligometastatic non-small cell lung cancer?Ann Transl Med. 2017 Mar;5(5):108. doi: 10.21037/atm.2017.01.21. Ann Transl Med. 2017. PMID: 28361073 Free PMC article. No abstract available.
-
Radical local therapy in combination with standard treatment for oligometastatic stage IV non-small-cell lung cancer.Ann Transl Med. 2017 Apr;5(7):165. doi: 10.21037/atm.2017.03.56. Ann Transl Med. 2017. PMID: 28480201 Free PMC article. No abstract available.
-
[Local consolidative therapy improves progression-free survival in patients with oligometastatic NSCLC : Results of a randomized phase II study].Strahlenther Onkol. 2017 Jul;193(7):595-597. doi: 10.1007/s00066-017-1147-4. Strahlenther Onkol. 2017. PMID: 28516198 German. No abstract available.
-
Perspectives on oligometastasis: challenges and opportunities.J Thorac Dis. 2018 Jan;10(1):113-117. doi: 10.21037/jtd.2017.12.77. J Thorac Dis. 2018. PMID: 29600035 Free PMC article. No abstract available.
-
Oligoreview of Non-Small Cell Lung Cancer Oligometastases.Int J Radiat Oncol Biol Phys. 2020 Mar 1;106(3):455-459. doi: 10.1016/j.ijrobp.2019.11.009. Int J Radiat Oncol Biol Phys. 2020. PMID: 32014142 No abstract available.
References
-
- Rusthoven KE, Hammerman SF, Kavanagh BD, Birtwhistle MJ, Stares M, Camidge DR. Is there a role for consolidative stereotactic body radiation therapy following first-line systemic therapy for metastatic lung cancer? A patterns-of-failure analysis. Acta Oncol. 2009;48(4):578–583. - PubMed
-
- Wong AC, Watson SP, Pitroda SP, Son CH, Das LC, Stack ME, et al. Clinical and molecular markers of long-term survival after oligometastasis-directed stereotactic body radiotherapy (SBRT) Cancer. 2016 - PubMed
-
- Salama JK, Chmura SJ, Mehta N, Yenice KM, Stadler WM, Vokes EE, et al. An initial report of a radiation dose-escalation trial in patients with one to five sites of metastatic disease. Clin Cancer Res. 2008;14(16):5255–5259. - PubMed
-
- Inoue T, Katoh N, Aoyama H, Onimaru R, Taguchi H, Onodera S, et al. Clinical outcomes of stereotactic brain and/or body radiotherapy for patients with oligometastatic lesions. Jpn J Clin Oncol. 2010;40(8):788–794. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous