β-Arrestin1 and Signal-transducing Adaptor Molecule 1 (STAM1) Cooperate to Promote Focal Adhesion Kinase Autophosphorylation and Chemotaxis via the Chemokine Receptor CXCR4
- PMID: 27789711
- PMCID: PMC5207078
- DOI: 10.1074/jbc.M116.757138
β-Arrestin1 and Signal-transducing Adaptor Molecule 1 (STAM1) Cooperate to Promote Focal Adhesion Kinase Autophosphorylation and Chemotaxis via the Chemokine Receptor CXCR4
Abstract
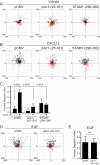
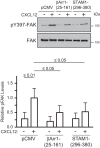
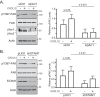
The chemokine receptor CXCR4 and its chemokine ligand CXCL12 mediate directed cell migration during organogenesis, immune responses, and metastatic disease. However, the mechanisms governing CXCL12/CXCR4-dependent chemotaxis remain poorly understood. Here, we show that the β-arrestin1·signal-transducing adaptor molecule 1 (STAM1) complex, initially identified to govern lysosomal trafficking of CXCR4, also mediates CXCR4-dependent chemotaxis. Expression of minigene fragments from β-arrestin1 or STAM1, known to disrupt the β-arrestin1·STAM1 complex, and RNAi against β-arrestin1 or STAM1, attenuates CXCL12-induced chemotaxis. The β-arrestin1·STAM1 complex is necessary for promoting autophosphorylation of focal adhesion kinase (FAK). FAK is necessary for CXCL12-induced chemotaxis and associates with and localizes with β-arrestin1 and STAM1 in a CXCL12-dependent manner. Our data reveal previously unknown roles in CXCR4-dependent chemotaxis for β-arrestin1 and STAM1, which we propose act in concert to regulate FAK signaling. The β-arrestin1·STAM1 complex is a promising target for blocking CXCR4-promoted FAK autophosphorylation and chemotaxis.
Keywords: C-X-C chemokine receptor type 4 (CXCR-4); CXCL12; G protein-coupled receptor (GPCR); PTK2 protein tyrosine kinase 2 (PTK2) (focal adhesion kinase) (FAK); STAM; chemokine; chemotaxis; β-arrestin.
© 2016 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures







References
-
- Ridley A. J. (2011) Life at the leading edge. Cell 145, 1012–1022 - PubMed
-
- Peled A., Petit I., Kollet O., Magid M., Ponomaryov T., Byk T., Nagler A., Ben-Hur H., Many A., Shultz L., Lider O., Alon R., Zipori D., and Lapidot T. (1999) Dependence of human stem cell engraftment and repopulation of NOD/SCID mice on CXCR4. Science 283, 845–848 - PubMed
-
- Tachibana K., Hirota S., Iizasa H., Yoshida H., Kawabata K., Kataoka Y., Kitamura Y., Matsushima K., Yoshida N., Nishikawa S., Kishimoto T., and Nagasawa T. (1998) The chemokine receptor CXCR4 is essential for vascularization of the gastrointestinal tract. Nature 393, 591–594 - PubMed
-
- Nagasawa T., Hirota S., Tachibana K., Takakura N., Nishikawa S., Kitamura Y., Yoshida N., Kikutani H., and Kishimoto T. (1996) Defects of B-cell lymphopoiesis and bone-marrow myelopoiesis in mice lacking the CXC chemokine PBSF/SDF-1. Nature 382, 635–638 - PubMed
-
- Zou Y. R., Kottmann A. H., Kuroda M., Taniuchi I., and Littman D. R. (1998) Function of the chemokine receptor CXCR4 in haematopoiesis and in cerebellar development. Nature 393, 595–599 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

