Selective Destruction of Interleukin 23-Induced Expansion of a Major Antigen-Specific γδ T-Cell Subset in Patients With Tuberculosis
- PMID: 27789724
- PMCID: PMC5853380
- DOI: 10.1093/infdis/jiw511
Selective Destruction of Interleukin 23-Induced Expansion of a Major Antigen-Specific γδ T-Cell Subset in Patients With Tuberculosis
Erratum in
-
Corrigendum.J Infect Dis. 2019 Oct 22;220(11):1862. doi: 10.1093/infdis/jiz384. J Infect Dis. 2019. PMID: 31613317 Free PMC article. No abstract available.
Abstract
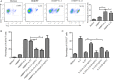
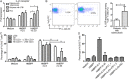
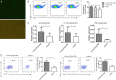
A loss of antigen-specific T-cell responses due to defective cytokine signaling during infections has not been reported. We hypothesize that tuberculosis can destroy signaling effects of selective cytokine(s) and induce exhaustion of antigen-specific T cells. To test this hypothesis, mechanistic studies were performed to examine whether and how tuberculosis blocked interleukin 23 (IL-23) and interleukin 2 (IL-2) signaling effects on a major human γδ T-cell subpopulation, phosphoantigen HMBPP-specific Vγ2Vδ2 T cells. IL-23 and IL-2 significantly expanded HMBPP-stimulated Vγ2Vδ2 T cells from subjects with latent tuberculosis infection, and IL-2 synergized the effect of IL-23. IL-23-induced expansion of Vγ2Vδ2 T cells involved STAT3. Surprisingly, patients with tuberculosis exhibited a selective destruction of IL-23-induced expansion of these cells. The tuberculosis-driven destruction of IL-23 signaling coincided with decreases of expression and phosphorylation of STAT3. Interestingly, impairing of STAT3 was linked to marked increases in the microRNAs (miRNAs) hsa-miR-337-3p and hsa-miR-125b-5p in Vγ2Vδ2 T cells from patients with tuberculosis. Downregulation of hsa-miR-337-3p and hsa-miR-125b-5p by miRNA sponges improved IL-23-mediated expansion of Vγ2Vδ2 T cells and restored the ability of these cells to produce anti-tuberculosis cytokines. These results support our hypothesis that tuberculosis can selectively impair a cytokine effect while sparing another and can induce exhaustion of T cells in response to the respective cytokine.
Keywords: JAK2/STAT3; T-cell exhaustion; Vγ2Vδ2 T cells; cytokine signaling; miRNA; tuberculosis.
© The Author 2016. Published by Oxford University Press for the Infectious Diseases Society of America. All rights reserved. For permissions, e-mail: journals.permissions@oup.com.
Figures



Similar articles
-
Th17-related cytokines contribute to recall-like expansion/effector function of HMBPP-specific Vγ2Vδ2 T cells after Mycobacterium tuberculosis infection or vaccination.Eur J Immunol. 2015 Feb;45(2):442-51. doi: 10.1002/eji.201444635. Eur J Immunol. 2015. PMID: 25141829 Free PMC article.
-
Tuberculosis-induced variant IL-4 mRNA encodes a cytokine functioning as growth factor for (E)-4-hydroxy-3-methyl-but-2-enyl pyrophosphate-specific Vgamma2Vdelta2 T cells.J Immunol. 2009 Jan 15;182(2):811-9. doi: 10.4049/jimmunol.182.2.811. J Immunol. 2009. PMID: 19124724 Free PMC article.
-
IL-12 Expands and Differentiates Human Vγ2Vδ2 T Effector Cells Producing Antimicrobial Cytokines and Inhibiting Intracellular Mycobacterial Growth.Front Immunol. 2019 Apr 26;10:913. doi: 10.3389/fimmu.2019.00913. eCollection 2019. Front Immunol. 2019. PMID: 31080452 Free PMC article.
-
Multifunctional immune responses of HMBPP-specific Vγ2Vδ2 T cells in M. tuberculosis and other infections.Cell Mol Immunol. 2013 Jan;10(1):58-64. doi: 10.1038/cmi.2012.46. Epub 2012 Nov 12. Cell Mol Immunol. 2013. PMID: 23147720 Free PMC article. Review.
-
Protective immune responses of major Vγ2Vδ2 T-cell subset in M. tuberculosis infection.Curr Opin Immunol. 2016 Oct;42:105-112. doi: 10.1016/j.coi.2016.06.005. Epub 2016 Aug 1. Curr Opin Immunol. 2016. PMID: 27491008 Free PMC article. Review.
Cited by
-
LncRNA SNHG16 Inhibits Intracellular M. tuberculosis Growth Involving Cathelicidin Pathway, Autophagy, and Effector Cytokines Production.ACS Omega. 2024 Oct 10;9(42):43115-43128. doi: 10.1021/acsomega.4c07053. eCollection 2024 Oct 22. ACS Omega. 2024. PMID: 39464459 Free PMC article.
-
From Host Defense to Metabolic Signatures: Unveiling the Role of γδ T Cells in Bacterial Infections.Biomolecules. 2024 Feb 15;14(2):225. doi: 10.3390/biom14020225. Biomolecules. 2024. PMID: 38397462 Free PMC article. Review.
-
Silencing miR-125b-5p attenuates inflammatory response and apoptosis inhibition in mycobacterium tuberculosis-infected human macrophages by targeting DNA damage-regulated autophagy modulator 2 (DRAM2).Cell Cycle. 2020 Nov;19(22):3182-3194. doi: 10.1080/15384101.2020.1838792. Epub 2020 Oct 30. Cell Cycle. 2020. PMID: 33121314 Free PMC article.
-
A CD4+CD161+ T-Cell Subset Present in Unexposed Humans, Not Tb Patients, Are Fast Acting Cells That Inhibit the Growth of Intracellular Mycobacteria Involving CD161 Pathway, Perforin, and IFN-γ/Autophagy.Front Immunol. 2021 Feb 26;12:599641. doi: 10.3389/fimmu.2021.599641. eCollection 2021. Front Immunol. 2021. PMID: 33732233 Free PMC article.
-
Functional Analysis of Genes in Mycobacterium tuberculosis Action Against Autophagosome-Lysosome Fusion.Indian J Microbiol. 2024 Jun;64(2):367-375. doi: 10.1007/s12088-024-01227-4. Epub 2024 Mar 7. Indian J Microbiol. 2024. PMID: 39011011 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

