Obesity, type 2 diabetes, and impaired insulin-stimulated blood flow: role of skeletal muscle NO synthase and endothelin-1
- PMID: 27789766
- PMCID: PMC5283852
- DOI: 10.1152/japplphysiol.00286.2016
Obesity, type 2 diabetes, and impaired insulin-stimulated blood flow: role of skeletal muscle NO synthase and endothelin-1
Abstract
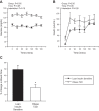
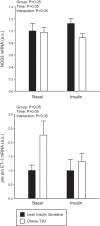
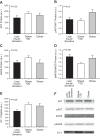
Increased endothelin-1 (ET-1) and reduced endothelial nitric oxide phosphorylation (peNOS) are hypothesized to reduce insulin-stimulated blood flow in type 2 diabetes (T2D), but studies examining these links in humans are limited. We sought to assess basal and insulin-stimulated endothelial signaling proteins (ET-1 and peNOS) in skeletal muscle from T2D patients. Ten obese T2D [glucose disposal rate (GDR): 6.6 ± 1.6 mg·kg lean body mass (LBM)-1·min-1] and 11 lean insulin-sensitive subjects (Lean GDR: 12.9 ± 1.2 mg·kg LBM-1·min-1) underwent a hyperinsulinemic-euglycemic clamp with vastus lateralis biopsies taken before and 60 min into the clamp. Basal biopsies were also taken in 11 medication-naïve, obese, non-T2D subjects. ET-1, peNOS (Ser1177), and eNOS protein and mRNA were measured from skeletal muscle samples containing native microvessels. Femoral artery blood flow was assessed by duplex Doppler ultrasound. Insulin-stimulated blood flow was reduced in obese T2D (Lean: +50.7 ± 6.5% baseline, T2D: +20.8 ± 5.2% baseline, P < 0.05). peNOS/eNOS content was higher in Lean under basal conditions and, although not increased by insulin, remained higher in Lean during the insulin clamp than in obese T2D (P < 0.05). ET-1 mRNA and peptide were 2.25 ± 0.50- and 1.52 ± 0.11-fold higher in obese T2D compared with Lean at baseline, and ET-1 peptide remained 2.02 ± 1.9-fold elevated in obese T2D after insulin infusion (P < 0.05) but did not increase with insulin in either group (P > 0.05). Obese non-T2D subjects tended to also display elevated basal ET-1 (P = 0.06). In summary, higher basal skeletal muscle expression of ET-1 and reduced peNOS/eNOS may contribute to a reduced insulin-stimulated leg blood flow response in obese T2D patients.
New & noteworthy: Although impairments in endothelial signaling are hypothesized to reduce insulin-stimulated blood flow in type 2 diabetes (T2D), human studies examining these links are limited. We provide the first measures of nitric oxide synthase and endothelin-1 expression from skeletal muscle tissue containing native microvessels in individuals with and without T2D before and during insulin stimulation. Higher basal skeletal muscle expression of endothelin-1 and reduced endothelial nitric oxide phosphorylation (peNOS)/eNOS may contribute to reduced insulin-stimulated blood flow in obese T2D patients.
Keywords: blood flow; endothelial signaling proteins; insulin resistance.
Copyright © 2017 the American Physiological Society.
Figures

References
-
- Baron AD, Brechtel-Hook G, Johnson A, Cronin J, Leaming R, Steinberg HO. Effect of perfusion rate on the time course of insulin-mediated skeletal muscle glucose uptake. Am J Physiol 271: E1067–E1072, 1996. - PubMed
-
- Barton M, Carmona R, Morawietz H, d’Uscio LV, Goettsch W, Hillen H, Haudenschild CC, Krieger JE, Münter K, Lattmann T, Lüscher TF, Shaw S. Obesity is associated with tissue-specific activation of renal angiotensin-converting enzyme in vivo: evidence for a regulatory role of endothelin. Hypertension 35: 329–336, 2000. doi:10.1161/01.HYP.35.1.329. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

