Influence of haem environment on the catalytic properties of the tetrathionate reductase TsdA from Campylobacter jejuni
- PMID: 27789780
- PMCID: PMC5146829
- DOI: 10.1042/BSR20160457
Influence of haem environment on the catalytic properties of the tetrathionate reductase TsdA from Campylobacter jejuni
Abstract
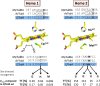
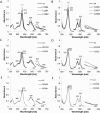
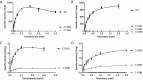
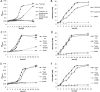
Bifunctional dihaem cytochrome c thiosulfate dehydrogenases/tetrathionate reductases (TsdA) exhibit different catalytic properties depending on the source organism. In the human food-borne intestinal pathogen Campylobacter jejuni, TsdA functions as a tetrathionate reductase enabling respiration with tetrathionate as an alternative electron acceptor. In the present study, evidence is provided that Cys138 and Met255 serve as the sixth ligands of Haem 1 and Haem 2 respectively, in the oxidized CjTsdA wt protein. Replacement of Cys138 resulted in a virtually inactive enzyme, confirming Haem 1 as the active site haem. Significantly, TsdA variants carrying amino acid exchanges in the vicinity of the electron-transferring Haem 2 (Met255, Asn254 and Lys252) exhibited markedly altered catalytic properties of the enzyme, showing these residues play a key role in the physiological function of TsdA. The growth phenotypes and tetrathionate reductase activities of a series of ΔtsdA/*tsdA complementation strains constructed in the original host C. jejuni 81116, showed that in vivo, the TsdA variants exhibited the same catalytic properties as the pure, recombinantly produced enzymes. However, variants that catalysed tetrathionate reduction more effectively than the wild-type enzyme did not allow better growth.
Keywords: axial haem ligation; cytochrome c; reaction directionality; tetrathionate reductase; thiosulfate.
© 2016 The Author(s).
Figures



Similar articles
-
Heme ligation and redox chemistry in two bacterial thiosulfate dehydrogenase (TsdA) enzymes.J Biol Chem. 2019 Nov 22;294(47):18002-18014. doi: 10.1074/jbc.RA119.010084. Epub 2019 Aug 29. J Biol Chem. 2019. PMID: 31467084 Free PMC article.
-
Tetrathionate stimulated growth of Campylobacter jejuni identifies a new type of bi-functional tetrathionate reductase (TsdA) that is widely distributed in bacteria.Mol Microbiol. 2013 Apr;88(1):173-88. doi: 10.1111/mmi.12176. Epub 2013 Mar 11. Mol Microbiol. 2013. PMID: 23421726
-
TsdC, a unique lipoprotein from Wolinella succinogenes that enhances tetrathionate reductase activity of TsdA.FEMS Microbiol Lett. 2017 Feb 1;364(3). doi: 10.1093/femsle/fnx003. FEMS Microbiol Lett. 2017. PMID: 28062520
-
The function, biogenesis and regulation of the electron transport chains in Campylobacter jejuni: New insights into the bioenergetics of a major food-borne pathogen.Adv Microb Physiol. 2019;74:239-329. doi: 10.1016/bs.ampbs.2019.02.003. Epub 2019 Mar 8. Adv Microb Physiol. 2019. PMID: 31126532 Review.
-
Structure and mechanism in the bacterial dihaem cytochrome c peroxidases.J Inorg Biochem. 2006 Apr;100(4):551-67. doi: 10.1016/j.jinorgbio.2005.12.008. Epub 2006 Jan 24. J Inorg Biochem. 2006. PMID: 16434100 Review.
Cited by
-
Reaction of Thiosulfate Dehydrogenase with a Substrate Mimic Induces Dissociation of the Cysteine Heme Ligand Giving Insights into the Mechanism of Oxidative Catalysis.J Am Chem Soc. 2022 Oct 12;144(40):18296-18304. doi: 10.1021/jacs.2c06062. Epub 2022 Sep 29. J Am Chem Soc. 2022. PMID: 36173876 Free PMC article.
-
Identification, Expression and Activity of Candidate Nitrite Reductases From Orange Beggiatoaceae, Guaymas Basin.Front Microbiol. 2019 Mar 29;10:644. doi: 10.3389/fmicb.2019.00644. eCollection 2019. Front Microbiol. 2019. PMID: 30984153 Free PMC article.
-
Heme ligation and redox chemistry in two bacterial thiosulfate dehydrogenase (TsdA) enzymes.J Biol Chem. 2019 Nov 22;294(47):18002-18014. doi: 10.1074/jbc.RA119.010084. Epub 2019 Aug 29. J Biol Chem. 2019. PMID: 31467084 Free PMC article.
References
-
- Oltman L.F., Claasen V.P., Kastelein P., Reijinders W.N.M., Stouthammer A.H. The influence of tungstate on the formation and activities of four reductases of Proteus mirabilis: identification of two new molybdoenzymes, chlorate reductase and tetrathionate reductase. FEBS Lett. 1979;106:43–46. doi: 10.1016/0014-5793(79)80691-2. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

