Treatment of systemic-onset juvenile arthritis with canakinumab
- PMID: 27790042
- PMCID: PMC5045123
- DOI: 10.2147/OARRR.S54215
Treatment of systemic-onset juvenile arthritis with canakinumab
Abstract
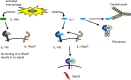
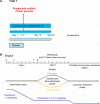
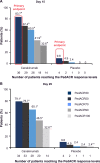
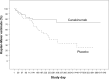
Treatment of systemic-onset juvenile idiopathic arthritis is challenging, but the availability of cytokine antagonists targeting interleukin-1 and interleukin-6 have markedly advanced the therapeutic options. In this review, we focus on the current experience with canakinumab, an interleukin-1 monoclonal human antibody for the treatment of systemic-onset juvenile idiopathic arthritis and describe its efficacy and safety. Canakinumab is an important, safe, and valid drug in the treatment of systemic-onset juvenile idiopathic arthritis.
Keywords: anakinra; canakinumab; interleukin-1; interleukin-6; systemic-onset juvenile idiopathic arthritis.
Figures
References
-
- Petty RE, Southwood TR, Baum J, et al. Revision of the proposed classification criteria for juvenile idiopathic arthritis: Durban, 1997. J Rheumatol. 1998;25:1991–1994. - PubMed
-
- Manners PJ, Bower C. Worldwide prevalence of juvenile arthritis why does it vary so much? J Rheumatol. 2002;29:1520–1530. - PubMed
-
- Prakken B, Albani S, Martini A. Juvenile idiopathic arthritis. Lancet. 2011;377:2138–2149. - PubMed
-
- Ravelli A, Martini A. Juvenile idiopathic arthritis. Lancet. 2007;369:767–778. - PubMed
-
- Martini A, Ravelli A, Di Fuccia G, Rosti V, Cazzola M, Barosi G. Intravenous iron therapy for severe anaemia in systemic-onset juvenile chronic arthritis. Lancet. 1994;344:1052–1054. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

