Changes in Serological Immunology Measures in UK and Kenyan Adults Post-controlled Human Malaria Infection
- PMID: 27790201
- PMCID: PMC5061779
- DOI: 10.3389/fmicb.2016.01604
Changes in Serological Immunology Measures in UK and Kenyan Adults Post-controlled Human Malaria Infection
Abstract
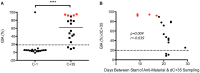
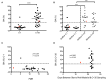
Background: The timing of infection is closely determined in controlled human malaria infection (CHMI) studies, and as such they provide a unique opportunity to dissect changes in immunological responses before and after a single infection. The first Kenyan Challenge Study (KCS) (Pan African Clinical Trial Registry: PACTR20121100033272) was performed in 2013 with the aim of establishing the CHMI model in Kenya. This study used aseptic, cryopreserved, attenuated Plasmodium falciparum sporozoites administered by needle and syringe (PfSPZ Challenge) and was the first to evaluate parasite dynamics post-CHMI in individuals with varying degrees of prior exposure to malaria. Methods: We describe detailed serological and functional immunological responses pre- and post-CHMI for participants in the KCS and compare these with those from malaria-naïve UK volunteers who also underwent CHMI (VAC049) (ClinicalTrials.gov NCT01465048) using PfSPZ Challenge. We assessed antibody responses to three key blood-stage merozoite antigens [merozoite surface protein 1 (MSP1), apical membrane protein 1 (AMA1), and reticulocyte-binding protein homolog 5 (RH5)] and functional activity using two candidate measures of anti-merozoite immunity; the growth inhibition activity (GIA) assay and the antibody-dependent respiratory burst activity (ADRB) assay. Results:Clear serological differences were observed pre- and post-CHMI by ELISA between malaria-naïve UK volunteers in VAC049, and Kenyan volunteers who had prior malaria exposure. Antibodies to AMA1 and schizont extract correlated with parasite multiplication rate (PMR) post-CHMI in KCS. Serum from volunteer 110 in KCS, who demonstrated a dramatically reduced PMR in vivo, had no in vitro GIA prior to CHMI but the highest level of ADRB activity. A significant difference in ADRB activity was seen between KCS volunteers with minimal and definite prior exposure to malaria and significant increases were seen in ADRB activity post-CHMI in Kenyan volunteers. Quinine and atovaquone/proguanil, previously assumed to be removed by IgG purification, were identified as likely giving rise to aberrantly high in vitro GIA results. Conclusions: The ADRB activity assay is a promising functional assay that warrants further investigation as a measure of prior exposure to malaria and predictor of control of parasite growth. The CHMI model can be used to evaluate potential measures of naturally-acquired immunity to malaria.
Keywords: ADRB; CHMI; ELISA; GIA; challenge; falciparum; immunity; malaria.
Figures



References
-
- Biswas S., Choudhary P., Elias S. C., Miura K., Milne K. H., de Cassan S. C., et al. . (2014). Assessment of humoral immune responses to blood-stage malaria antigens following ChAd63-MVA immunization, controlled human malaria infection and natural exposure. PLoS ONE 9:e107903. 10.1371/journal.pone.0107903 - DOI - PMC - PubMed
-
- Douglas A. D., Baldeviano G. C., Lucas C. M., Lugo-Roman L. A., Crosnier C., Bartholdson S. J., et al. . (2015). A PfRH5-based vaccine is efficacious against heterologous strain blood-stage Plasmodium falciparum infection in aotus monkeys. Cell Host Microbe 17, 130–139. 10.1016/j.chom.2014.11.017 - DOI - PMC - PubMed
-
- Douglas A. D., Edwards N. J., Duncan C. J., Thompson F. M., Sheehy S. H., O'hara G. A., et al. . (2013). Comparison of modeling methods to determine liver-to-blood inocula and parasite multiplication rates during controlled human malaria infection. J. Infect. Dis. 208, 340–345. 10.1093/infdis/jit156 - DOI - PMC - PubMed
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

