SpyB, a Small Heme-Binding Protein, Affects the Composition of the Cell Wall in Streptococcus pyogenes
- PMID: 27790410
- PMCID: PMC5061733
- DOI: 10.3389/fcimb.2016.00126
SpyB, a Small Heme-Binding Protein, Affects the Composition of the Cell Wall in Streptococcus pyogenes
Abstract
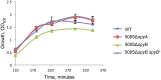
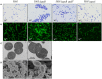
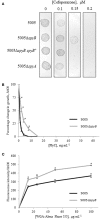
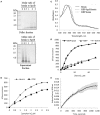
Streptococcus pyogenes (Group A Streptococcus or GAS) is a hemolytic human pathogen associated with a wide variety of infections ranging from minor skin and throat infections to life-threatening invasive diseases. The cell wall of GAS consists of peptidoglycan sacculus decorated with a carbohydrate comprising a polyrhamnose backbone with immunodominant N-acetylglucosamine side-chains. All GAS genomes contain the spyBA operon, which encodes a 35-amino-acid membrane protein SpyB, and a membrane-bound C3-like ADP-ribosyltransferase SpyA. In this study, we addressed the function of SpyB in GAS. Phenotypic analysis of a spyB deletion mutant revealed increased bacterial aggregation, and reduced sensitivity to β-lactams of the cephalosporin class and peptidoglycan hydrolase PlyC. Glycosyl composition analysis of cell wall isolated from the spyB mutant suggested an altered carbohydrate structure compared with the wild-type strain. Furthermore, we found that SpyB associates with heme and protoporphyrin IX. Heme binding induces SpyB dimerization, which involves disulfide bond formation between the subunits. Thus, our data suggest the possibility that SpyB activity is regulated by heme.
Keywords: ADP-ribosyltransferase; Group A carbohydrate; SpyA; SpyB; Streptococcus pyogenes; cell wall; heme.
Figures

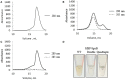
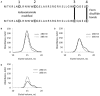
References
-
- Ascoli F., Fanelli M. R., Antonini E. (1980). Preparation and properties of apohemoglobin and reconstituted hemoglobins. Methods Enzymol. 76, 72–87. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

