Sending Out an SOS: Mitochondria as a Signaling Hub
- PMID: 27790613
- PMCID: PMC5061732
- DOI: 10.3389/fcell.2016.00109
Sending Out an SOS: Mitochondria as a Signaling Hub
Abstract
Normal cellular physiology is critically dependent on numerous mitochondrial activities including energy conversion, cofactor and precursor metabolite synthesis, and regulation of ion and redox homeostasis. Advances in mitochondrial research during the last two decades provide solid evidence that these organelles are deeply integrated with the rest of the cell and multiple mechanisms are in place to monitor and communicate functional states of mitochondria. In many cases, however, the exact molecular nature of various mitochondria-to-cell communication pathways is only beginning to emerge. Here, we review various signals emitted by distressed or dysfunctional mitochondria and the stress-responsive pathways activated in response to these signals in order to restore mitochondrial function and promote cellular survival.
Keywords: metabolites; mitochondria; mitochondrial stress; reactive oxygen species; retrograde signaling.
Figures
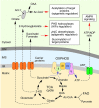
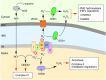
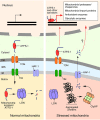

References
-
- Adam J., Hatipoglu E., O'Flaherty L., Ternette N., Sahgal N., Lockstone H., et al. (2011). Renal cyst formation ih Fh1-deficient mice is independent of the Hif/Phd pathway: roles for fumarate in KEAP1 succination and Nrf2 signaling. Cancer Cell 20, 524–537. 10.1016/j.ccr.2011.09.006 - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

