Actinobacillus pleuropneumoniae grows as aggregates in the lung of pigs: is it time to refine our in vitro biofilm assays?
- PMID: 27790837
- PMCID: PMC5481545
- DOI: 10.1111/1751-7915.12432
Actinobacillus pleuropneumoniae grows as aggregates in the lung of pigs: is it time to refine our in vitro biofilm assays?
Abstract
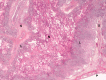
Actinobacillus pleuropneumoniae causes porcine pleuropneumonia and forms biofilms in vitro on abiotic surfaces; however, presence of biofilms during infections has not been documented. The aim of this study was to use a species-specific fluorescent oligonucleotide probe and confocal microscopy to localize A. pleuropneumoniae in the lungs of two naturally infected pigs. Actinobacillus pleuropneumoniae was detected by fluorescence in situ hybridization and observed to grow as aggregates (~30-45 μm) during a natural infection. As the A. pleuropneumoniae aggregates observed in porcine lungs differed from the biofilms grown on a solid surface obtained in vitro, we designed a new biofilm assay using agarose, a porous substrate, favouring the formation of aggregates. In this study, we described for the first time the mode of growth of A. pleuropneumoniae during a natural infection in pigs. We also propose an in vitro biofilm assay for A. pleuropneumoniae using a porous substrate which allows the formation of aggregates. This assay might be more representative of the in vivo situation, at least in terms of the size of the bacterial aggregates and the presence of a porous matrix, and could potentially be used to test the susceptibility of A. pleuropneumoniae aggregates to antibiotics and disinfectants.
© 2016 The Authors. Microbial Biotechnology published by John Wiley & Sons Ltd and Society for Applied Microbiology.
Figures



References
-
- Archambault, M. , Harel, J. , Gouré, J. , Tremblay, Y.D. , and Jacques, M. (2012) Antimicrobial susceptibilities and resistance genes of Canadian isolates of Actinobacillus pleuropneumoniae . Microb Drug Resist 18: 198–206. - PubMed
-
- Bjarnsholt, T. , Alhede, M. , Eickhardt‐Sorensen, S.R. , Moser, C. , Kühl, M. , Jensen, P.O. , and Hoiby, N. (2013) The in vivo biofilm. Trends Microbiol 21: 466–474. - PubMed
-
- Bossé, J.T. , Janson, H. , Sheehan, B.J. , Beddek, A.J. , Rycroft, A.N. , Kroll, J.S. , and Langford, P.R. (2002) Actinobacillus pleuropneumoniae: pathobiology and pathogenesis of infection. Microbes Infect 4: 225–235. - PubMed
-
- Costerton, J.W. , Stewart, P.S. , and Greenberg, E.P. (1999) Bacterial biofilms: a common cause of persistent infections. Science 284: 1318–1322. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

