Proteolytic processing of Middle East respiratory syndrome coronavirus spikes expands virus tropism
- PMID: 27791014
- PMCID: PMC5086990
- DOI: 10.1073/pnas.1608147113
Proteolytic processing of Middle East respiratory syndrome coronavirus spikes expands virus tropism
Abstract
Middle East respiratory syndrome coronavirus (MERS-CoV) infects humans from zoonotic sources and causes severe pulmonary disease. Virions require spike (S) glycoproteins for binding to cell receptors and for catalyzing virus-cell membrane fusion. Fusion occurs only after S proteins are cleaved sequentially, first during their secretion through the exocytic organelles of virus-producing cells, and second after virus binding to target-cell receptors. To more precisely determine how sequential proteolysis contributes to CoV infection, we introduced S mutations obstructing the first cleavages. These mutations severely compromised MERS-CoV infection into human lung-derived cells, but had little effect on infection into several other cell types. These cell type-specific requirements for proteolysis correlated with S conformations during cell entry. Without the first cleavages, S proteins resisted cell receptor-induced conformational changes, which restricted the second, fusion-activating cleavages. Consistent with these findings, precleaved MERS viruses used receptor-proximal, cell-surface proteases to effect the second fusion-activating cleavages during cell entry, whereas the more rigid uncleaved MERS viruses trafficked past these cell-surface proteases and into endosomes. Uncleaved viruses were less infectious to human airway epithelial and Calu3 cell cultures because they lacked sufficient endosomal fusion-activating proteases. Thus, by sensitizing viruses to receptor-induced conformational changes, the first S cleavages expand virus tropism to cell types that are relevant to lung infection, and therefore may be significant determinants of MERS-CoV virulence.
Keywords: coronavirus; protease; receptor; virus entry.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

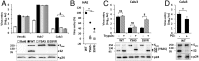


References
-
- Kido H, et al. Role of host cellular proteases in the pathogenesis of influenza and influenza-induced multiple organ failure. Biochim Biophys Acta. 2012;1824(1):186–194. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

