Functional connectivity with the retrosplenial cortex predicts cognitive aging in rats
- PMID: 27791017
- PMCID: PMC5087009
- DOI: 10.1073/pnas.1525309113
Functional connectivity with the retrosplenial cortex predicts cognitive aging in rats
Abstract
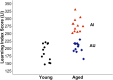
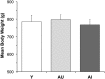
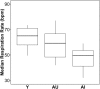
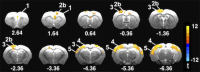
Changes in the functional connectivity (FC) of large-scale brain networks are a prominent feature of brain aging, but defining their relationship to variability along the continuum of normal and pathological cognitive outcomes has proved challenging. Here we took advantage of a well-characterized rat model that displays substantial individual differences in hippocampal memory during aging, uncontaminated by slowly progressive, spontaneous neurodegenerative disease. By this approach, we aimed to interrogate the underlying neural network substrates that mediate aging as a uniquely permissive condition and the primary risk for neurodegeneration. Using resting state (rs) blood oxygenation level-dependent fMRI and a restrosplenial/posterior cingulate cortex seed, aged rats demonstrated a large-scale network that had a spatial distribution similar to the default mode network (DMN) in humans, consistent with earlier findings in younger animals. Between-group whole brain contrasts revealed that aged subjects with documented deficits in memory (aged impaired) displayed widespread reductions in cortical FC, prominently including many areas outside the DMN, relative to both young adults (Y) and aged rats with preserved memory (aged unimpaired, AU). Whereas functional connectivity was relatively preserved in AU rats, they exhibited a qualitatively distinct network signature, comprising the loss of an anticorrelated network observed in Y adults. Together the findings demonstrate that changes in rs-FC are specifically coupled to variability in the cognitive outcome of aging, and that successful neurocognitive aging is associated with adaptive remodeling, not simply the persistence of youthful network dynamics.
Keywords: default mode network; functional connectivity; neurocognitive aging; rat model; resting-state fMRI.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: Anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. - PubMed
-
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34(4):537–541. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

