Nanomechanical mechanism for lipid bilayer damage induced by carbon nanotubes confined in intracellular vesicles
- PMID: 27791073
- PMCID: PMC5098676
- DOI: 10.1073/pnas.1605030113
Nanomechanical mechanism for lipid bilayer damage induced by carbon nanotubes confined in intracellular vesicles
Abstract
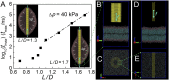
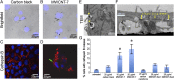
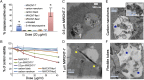
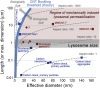
Understanding the behavior of low-dimensional nanomaterials confined in intracellular vesicles has been limited by the resolution of bioimaging techniques and the complex nature of the problem. Recent studies report that long, stiff carbon nanotubes are more cytotoxic than flexible varieties, but the mechanistic link between stiffness and cytotoxicity is not understood. Here we combine analytical modeling, molecular dynamics simulations, and in vitro intracellular imaging methods to reveal 1D carbon nanotube behavior within intracellular vesicles. We show that stiff nanotubes beyond a critical length are compressed by lysosomal membranes causing persistent tip contact with the inner membrane leaflet, leading to lipid extraction, lysosomal permeabilization, release of cathepsin B (a lysosomal protease) into the cytoplasm, and cell death. The precise material parameters needed to activate this unique mechanical pathway of nanomaterials interaction with intracellular vesicles were identified through coupled modeling, simulation, and experimental studies on carbon nanomaterials with wide variation in size, shape, and stiffness, leading to a generalized classification diagram for 1D nanocarbons that distinguishes pathogenic from biocompatible varieties based on a nanomechanical buckling criterion. For a wide variety of other 1D material classes (metal, oxide, polymer), this generalized classification diagram shows a critical threshold in length/width space that represents a transition from biologically soft to stiff, and thus identifies the important subset of all 1D materials with the potential to induce lysosomal permeability by the nanomechanical mechanism under investigation.
Keywords: biomembrane; lipid extraction; lysosomal permeabilization; molecular dynamics; one-dimensional nanomaterials.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Tu Y, et al. Destructive extraction of phospholipids from Escherichia coli membranes by graphene nanosheets. Nat Nanotechnol. 2013;8(8):594–601. - PubMed
-
- Vecitis CD, Zodrow KR, Kang S, Elimelech M. Electronic-structure-dependent bacterial cytotoxicity of single-walled carbon nanotubes. ACS Nano. 2010;4(9):5471–5479. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

