Inositol polyphosphates intersect with signaling and metabolic networks via two distinct mechanisms
- PMID: 27791083
- PMCID: PMC5098652
- DOI: 10.1073/pnas.1606853113
Inositol polyphosphates intersect with signaling and metabolic networks via two distinct mechanisms
Abstract
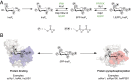
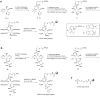
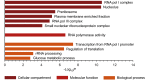
Inositol-based signaling molecules are central eukaryotic messengers and include the highly phosphorylated, diffusible inositol polyphosphates (InsPs) and inositol pyrophosphates (PP-InsPs). Despite the essential cellular regulatory functions of InsPs and PP-InsPs (including telomere maintenance, phosphate sensing, cell migration, and insulin secretion), the majority of their protein targets remain unknown. Here, the development of InsP and PP-InsP affinity reagents is described to comprehensively annotate the interactome of these messenger molecules. By using the reagents as bait, >150 putative protein targets were discovered from a eukaryotic cell lysate (Saccharomyces cerevisiae). Gene Ontology analysis of the binding partners revealed a significant overrepresentation of proteins involved in nucleotide metabolism, glucose metabolism, ribosome biogenesis, and phosphorylation-based signal transduction pathways. Notably, we isolated and characterized additional substrates of protein pyrophosphorylation, a unique posttranslational modification mediated by the PP-InsPs. Our findings not only demonstrate that the PP-InsPs provide a central line of communication between signaling and metabolic networks, but also highlight the unusual ability of these molecules to access two distinct modes of action.
Keywords: affinity reagents; inositol pyrophosphates; metabolism; protein pyrophosphorylation; signal transduction.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
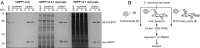
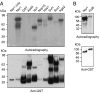
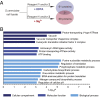
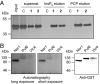
References
-
- Wellen KE, Thompson CB. A two-way street: Reciprocal regulation of metabolism and signalling. Nat Rev Mol Cell Biol. 2012;13(4):270–276. - PubMed
-
- Verdin E, Ott M. 50 years of protein acetylation: From gene regulation to epigenetics, metabolism and beyond. Nat Rev Mol Cell Biol. 2015;16(4):258–264. - PubMed
-
- Wilson MSC, Livermore TM, Saiardi A. Inositol pyrophosphates: Between signalling and metabolism. Biochem J. 2013;452(3):369–379. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

