Extracellular RNA is transported from one generation to the next in Caenorhabditis elegans
- PMID: 27791108
- PMCID: PMC5098612
- DOI: 10.1073/pnas.1608959113
Extracellular RNA is transported from one generation to the next in Caenorhabditis elegans
Abstract
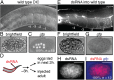
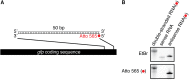
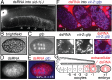
Experiences during the lifetime of an animal have been proposed to have consequences for subsequent generations. Although it is unclear how such intergenerational transfer of information occurs, RNAs found extracellularly in animals are candidate molecules that can transfer gene-specific regulatory information from one generation to the next because they can enter cells and regulate gene expression. In support of this idea, when double-stranded RNA (dsRNA) is introduced into some animals, the dsRNA can silence genes of matching sequence and the silencing can persist in progeny. Such persistent gene silencing is thought to result from sequence-specific interaction of the RNA within parents to generate chromatin modifications, DNA methylation, and/or secondary RNAs, which are then inherited by progeny. Here, we show that dsRNA can be directly transferred between generations in the worm Caenorhabditis elegans Intergenerational transfer of dsRNA occurs even in animals that lack any DNA of matching sequence, and dsRNA that reaches progeny can spread between cells to cause gene silencing. Surprisingly, extracellular dsRNA can also reach progeny without entry into the cytosol, presumably within intracellular vesicles. Fluorescently labeled dsRNA is imported from extracellular space into oocytes along with yolk and accumulates in punctate structures within embryos. Subsequent entry into the cytosol of early embryos causes gene silencing in progeny. These results demonstrate the transport of extracellular RNA from one generation to the next to regulate gene expression in an animal and thus suggest a mechanism for the transmission of experience-dependent effects between generations.
Keywords: circulating RNA; endocytosis; epigenetics; parental RNAi; transgenerational inheritance.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

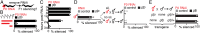
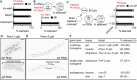

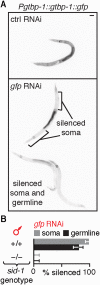

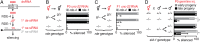






References
-
- Pembrey ME, et al. ALSPAC Study Team Sex-specific, male-line transgenerational responses in humans. Eur J Hum Genet. 2006;14(2):159–166. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

