Archaea catalyze iron-dependent anaerobic oxidation of methane
- PMID: 27791118
- PMCID: PMC5111651
- DOI: 10.1073/pnas.1609534113
Archaea catalyze iron-dependent anaerobic oxidation of methane
Abstract
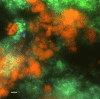
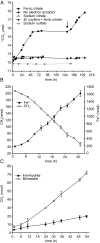
Anaerobic oxidation of methane (AOM) is crucial for controlling the emission of this potent greenhouse gas to the atmosphere. Nitrite-, nitrate-, and sulfate-dependent methane oxidation is well-documented, but AOM coupled to the reduction of oxidized metals has so far been demonstrated only in environmental samples. Here, using a freshwater enrichment culture, we show that archaea of the order Methanosarcinales, related to "Candidatus Methanoperedens nitroreducens," couple the reduction of environmentally relevant forms of Fe3+ and Mn4+ to the oxidation of methane. We obtained an enrichment culture of these archaea under anaerobic, nitrate-reducing conditions with a continuous supply of methane. Via batch incubations using [13C]methane, we demonstrated that soluble ferric iron (Fe3+, as Fe-citrate) and nanoparticulate forms of Fe3+ and Mn4+ supported methane-oxidizing activity. CO2 and ferrous iron (Fe2+) were produced in stoichiometric amounts. Our study connects the previous finding of iron-dependent AOM to microorganisms detected in numerous habitats worldwide. Consequently, it enables a better understanding of the interaction between the biogeochemical cycles of iron and methane.
Keywords: anaerobic oxidation of methane; archaea; iron reduction; manganese reduction; multiheme proteins.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Comment in
-
Metal Oxide Reduction Linked to Anaerobic Methane Oxidation.Trends Microbiol. 2017 Feb;25(2):88-90. doi: 10.1016/j.tim.2016.12.001. Epub 2016 Dec 13. Trends Microbiol. 2017. PMID: 27986381
References
-
- Trotsenko YA, Murrell JC. Metabolic aspects of aerobic obligate methanotrophy. In: Laskin AI, Sariaslani S, Gadd GM, editors. Advances in Applied Microbiology. Vol 63. Academic; Cambridge, MA: 2008. pp. 183–229. - PubMed
-
- Knittel K, Boetius A. Anaerobic oxidation of methane: Progress with an unknown process. Annu Rev Microbiol. 2009;63:311–334. - PubMed
-
- Myhre G, et al. Anthropogenic and natural radiative forcing. In: Stocker TF, et al., editors. Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge Univ Press; Cambridge, UK: 2014. pp. 659–740.
-
- Milucka J, et al. Zero-valent sulphur is a key intermediate in marine methane oxidation. Nature. 2012;491(7425):541–546. - PubMed
-
- Haroon MF, et al. Anaerobic oxidation of methane coupled to nitrate reduction in a novel archaeal lineage. Nature. 2013;500(7464):567–570. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

