An evolutionary conserved Hexim1 peptide binds to the Cdk9 catalytic site to inhibit P-TEFb
- PMID: 27791144
- PMCID: PMC5111705
- DOI: 10.1073/pnas.1612331113
An evolutionary conserved Hexim1 peptide binds to the Cdk9 catalytic site to inhibit P-TEFb
Abstract
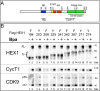
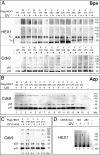
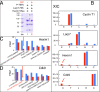
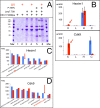
The positive transcription elongation factor (P-TEFb) is required for the transcription of most genes by RNA polymerase II. Hexim proteins associated with 7SK RNA bind to P-TEFb and reversibly inhibit its activity. P-TEFb comprises the Cdk9 cyclin-dependent kinase and a cyclin T. Hexim proteins have been shown to bind the cyclin T subunit of P-TEFb. How this binding leads to inhibition of the kinase activity of Cdk9 has remained elusive, however. Using a photoreactive amino acid incorporated into proteins, we show that in live cells, cell extracts, and in vitro reconstituted complexes, Hexim1 cross-links and thus contacts Cdk9. Notably, replacement of a phenylalanine, F208, belonging to an evolutionary conserved Hexim1 peptide (202PYNTTQFLM210) known as the "PYNT" sequence, cross-links a peptide within the activation segment that controls access to the Cdk9 catalytic cleft. Reciprocally, Hexim1 is cross-linked by a photoreactive amino acid replacing Cdk9 W193, a tryptophan within this activation segment. These findings provide evidence of a direct interaction between Cdk9 and its inhibitor, Hexim1. Based on similarities with Cdk2 3D structure, the Cdk9 peptide cross-linked by Hexim1 corresponds to the substrate binding-site. Accordingly, the Hexim1 PYNT sequence is proposed to interfere with substrate binding to Cdk9 and thereby to inhibit its kinase activity.
Keywords: benzoyl phenylalanine; cyclin-dependent kinase inhibition; protein–protein cross-linking; regulatory noncoding RNA; transcription factor regulation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Binding of the 7SK snRNA turns the HEXIM1 protein into a P-TEFb (CDK9/cyclin T) inhibitor.EMBO J. 2004 Jul 7;23(13):2608-19. doi: 10.1038/sj.emboj.7600275. Epub 2004 Jun 17. EMBO J. 2004. PMID: 15201869 Free PMC article.
-
Analysis of the large inactive P-TEFb complex indicates that it contains one 7SK molecule, a dimer of HEXIM1 or HEXIM2, and two P-TEFb molecules containing Cdk9 phosphorylated at threonine 186.J Biol Chem. 2005 Aug 5;280(31):28819-26. doi: 10.1074/jbc.M502712200. Epub 2005 Jun 17. J Biol Chem. 2005. PMID: 15965233
-
A human immunodeficiency virus type 1 Tat-like arginine-rich RNA-binding domain is essential for HEXIM1 to inhibit RNA polymerase II transcription through 7SK snRNA-mediated inactivation of P-TEFb.Mol Cell Biol. 2004 Jun;24(12):5094-105. doi: 10.1128/MCB.24.12.5094-5105.2004. Mol Cell Biol. 2004. PMID: 15169877 Free PMC article.
-
RNA-driven cyclin-dependent kinase regulation: when CDK9/cyclin T subunits of P-TEFb meet their ribonucleoprotein partners.Biotechnol J. 2008 Aug;3(8):1022-32. doi: 10.1002/biot.200800104. Biotechnol J. 2008. PMID: 18655042 Review.
-
The 7SK snRNP complex: a critical regulator in carcinogenesis.Biochimie. 2025 May 12:S0300-9084(25)00084-7. doi: 10.1016/j.biochi.2025.05.003. Online ahead of print. Biochimie. 2025. PMID: 40368082 Review.
Cited by
-
Keep calm and reboot - how cells restart transcription after DNA damage and DNA repair.FEBS Lett. 2025 Jan;599(2):275-294. doi: 10.1002/1873-3468.14964. Epub 2024 Jul 11. FEBS Lett. 2025. PMID: 38991979 Free PMC article. Review.
-
An alpha-herpesvirus employs host HEXIM1 to promote viral transcription.J Virol. 2024 Mar 19;98(3):e0139223. doi: 10.1128/jvi.01392-23. Epub 2024 Feb 16. J Virol. 2024. PMID: 38363111 Free PMC article.
-
CSB-Dependent Cyclin-Dependent Kinase 9 Degradation and RNA Polymerase II Phosphorylation during Transcription-Coupled Repair.Mol Cell Biol. 2019 Mar 1;39(6):e00225-18. doi: 10.1128/MCB.00225-18. Print 2019 Mar 15. Mol Cell Biol. 2019. PMID: 30602496 Free PMC article.
-
The cell biology of HIV-1 latency and rebound.Retrovirology. 2024 Apr 5;21(1):6. doi: 10.1186/s12977-024-00639-w. Retrovirology. 2024. PMID: 38580979 Free PMC article. Review.
-
7SK small nuclear RNA, a multifunctional transcriptional regulatory RNA with gene-specific features.Transcription. 2018;9(2):95-101. doi: 10.1080/21541264.2017.1344346. Epub 2017 Oct 4. Transcription. 2018. PMID: 28820318 Free PMC article.
References
-
- Nguyen VT, Kiss T, Michels AA, Bensaude O. 7SK small nuclear RNA binds to and inhibits the activity of CDK9/cyclin T complexes. Nature. 2001;414(6861):322–325. - PubMed
-
- Yang Z, Zhu Q, Luo K, Zhou Q. The 7SK small nuclear RNA inhibits the CDK9/cyclin T1 kinase to control transcription. Nature. 2001;414(6861):317–322. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

