Exogenous Melatonin Improves Plant Iron Deficiency Tolerance via Increased Accumulation of Polyamine-Mediated Nitric Oxide
- PMID: 27792144
- PMCID: PMC5133778
- DOI: 10.3390/ijms17111777
Exogenous Melatonin Improves Plant Iron Deficiency Tolerance via Increased Accumulation of Polyamine-Mediated Nitric Oxide
Abstract
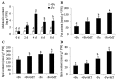
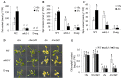
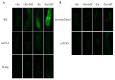
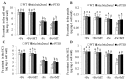
Melatonin has recently been demonstrated to play important roles in the regulation of plant growth, development, and abiotic and biotic stress responses. However, the possible involvement of melatonin in Fe deficiency responses and the underlying mechanisms remained elusive in Arabidopsis thaliana. In this study, Fe deficiency quickly induced melatonin synthesis in Arabidopsis plants. Exogenous melatonin significantly increased the soluble Fe content of shoots and roots, and decreased the levels of root cell wall Fe bound to pectin and hemicellulose, thus alleviating Fe deficiency-induced chlorosis. Intriguingly, melatonin treatments induced a significant increase of nitric oxide (NO) accumulation in roots of Fe-deficient plants, but not in those of polyamine-deficient (adc2-1 and d-arginine-treated) plants. Moreover, the melatonin-alleviated leaf chlorosis was blocked in the polyamine- and NO-deficient (nia1nia2noa1 and c-PTIO-treated) plants, and the melatonin-induced Fe remobilization was largely inhibited. In addition, the expression of some Fe acquisition-related genes, including FIT1, FRO2, and IRT1 were significantly up-regulated by melatonin treatments, whereas the enhanced expression of these genes was obviously suppressed in the polyamine- and NO-deficient plants. Collectively, our results provide evidence to support the view that melatonin can increase the tolerance of plants to Fe deficiency in a process dependent on the polyamine-induced NO production under Fe-deficient conditions.
Keywords: iron deficiency; iron remobilization; melatonin; nitric oxide (NO); polyamine.
Conflict of interest statement
The authors declare no conflict of interest. The funding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
Figures






Similar articles
-
Glutathione plays an essential role in nitric oxide-mediated iron-deficiency signaling and iron-deficiency tolerance in Arabidopsis.Plant J. 2015 Nov;84(3):464-77. doi: 10.1111/tpj.13011. Plant J. 2015. PMID: 26333047
-
Putrescine Alleviates Iron Deficiency via NO-Dependent Reutilization of Root Cell-Wall Fe in Arabidopsis.Plant Physiol. 2016 Jan;170(1):558-67. doi: 10.1104/pp.15.01617. Epub 2015 Nov 17. Plant Physiol. 2016. PMID: 26578707 Free PMC article.
-
The essential basic helix-loop-helix protein FIT1 is required for the iron deficiency response.Plant Cell. 2004 Dec;16(12):3400-12. doi: 10.1105/tpc.104.024315. Epub 2004 Nov 11. Plant Cell. 2004. PMID: 15539473 Free PMC article.
-
Molecular mechanisms governing Arabidopsis iron uptake.Trends Plant Sci. 2015 Feb;20(2):124-33. doi: 10.1016/j.tplants.2014.11.004. Epub 2014 Dec 8. Trends Plant Sci. 2015. PMID: 25499025 Review.
-
Physiological and molecular mechanisms of plant-root responses to iron toxicity.J Plant Physiol. 2024 Jun;297:154257. doi: 10.1016/j.jplph.2024.154257. Epub 2024 Apr 22. J Plant Physiol. 2024. PMID: 38688043 Review.
Cited by
-
Melatonin Modulates Plant Tolerance to Heavy Metal Stress: Morphological Responses to Molecular Mechanisms.Int J Mol Sci. 2021 Oct 23;22(21):11445. doi: 10.3390/ijms222111445. Int J Mol Sci. 2021. PMID: 34768875 Free PMC article. Review.
-
New insights on neurotransmitters signaling mechanisms in plants.Plant Signal Behav. 2020 Jun 2;15(6):1737450. doi: 10.1080/15592324.2020.1737450. Epub 2020 May 6. Plant Signal Behav. 2020. PMID: 32375557 Free PMC article. Review.
-
ROS and NO Regulation by Melatonin Under Abiotic Stress in Plants.Antioxidants (Basel). 2020 Nov 3;9(11):1078. doi: 10.3390/antiox9111078. Antioxidants (Basel). 2020. PMID: 33153156 Free PMC article. Review.
-
Involvement of Nitric Oxide and Melatonin Enhances Cadmium Resistance of Tomato Seedlings through Regulation of the Ascorbate-Glutathione Cycle and ROS Metabolism.Int J Mol Sci. 2023 May 31;24(11):9526. doi: 10.3390/ijms24119526. Int J Mol Sci. 2023. PMID: 37298477 Free PMC article.
-
Nitric Oxide Is Essential for Melatonin to Enhance Nitrate Tolerance of Cucumber Seedlings.Molecules. 2022 Sep 7;27(18):5806. doi: 10.3390/molecules27185806. Molecules. 2022. PMID: 36144541 Free PMC article.
References
-
- Lerner A.B., Case J.D., Takahashi Y., Lee T.H., Mori W. Isolation of melatonin, a pineal factor that lightens melanocytes. J. Am. Chem. Soc. 1958;80 doi: 10.1021/ja01543a060. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

