O2 Activation by Non-Heme Iron Enzymes
- PMID: 27792301
- PMCID: PMC5345855
- DOI: 10.1021/acs.biochem.6b00635
O2 Activation by Non-Heme Iron Enzymes
Abstract
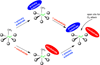
The non-heme Fe enzymes are ubiquitous in nature and perform a wide range of functions involving O2 activation. These had been difficult to study relative to heme enzymes; however, spectroscopic methods that provide significant insight into the correlation of structure with function have now been developed. This Current Topics article summarizes both the molecular mechanism these enzymes use to control O2 activation in the presence of cosubstrates and the oxygen intermediates these reactions generate. Three types of O2 activation are observed. First, non-heme reactivity is shown to be different from heme chemistry where a low-spin FeIII-OOH non-heme intermediate directly reacts with substrate. Also, two subclasses of non-heme Fe enzymes generate high-spin FeIV═O intermediates that provide both σ and π frontier molecular orbitals that can control selectivity. Finally, for several subclasses of non-heme Fe enzymes, binding of the substrate to the FeII site leads to the one-electron reductive activation of O2 to an FeIII-superoxide capable of H atom abstraction and electrophilic attack.
Figures

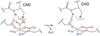





Similar articles
-
Geometric and electronic structure contributions to function in non-heme iron enzymes.Acc Chem Res. 2013 Nov 19;46(11):2725-39. doi: 10.1021/ar400149m. Epub 2013 Sep 26. Acc Chem Res. 2013. PMID: 24070107 Free PMC article.
-
Stereospecific alkane hydroxylation by non-heme iron catalysts: mechanistic evidence for an Fe(V)=O active species.J Am Chem Soc. 2001 Jul 4;123(26):6327-37. doi: 10.1021/ja010310x. J Am Chem Soc. 2001. PMID: 11427057
-
Mechanistic insights on the ortho-hydroxylation of aromatic compounds by non-heme iron complex: a computational case study on the comparative oxidative ability of ferric-hydroperoxo and high-valent Fe(IV)═O and Fe(V)═O intermediates.J Am Chem Soc. 2013 Mar 20;135(11):4235-49. doi: 10.1021/ja307077f. Epub 2013 Mar 7. J Am Chem Soc. 2013. PMID: 23373840
-
Quantum chemical studies of dioxygen activation by mononuclear non-heme iron enzymes with the 2-His-1-carboxylate facial triad.Dalton Trans. 2004 Oct 21;(20):3153-62. doi: 10.1039/B408340G. Epub 2004 Aug 27. Dalton Trans. 2004. PMID: 15483690 Review.
-
Structure/function correlations over binuclear non-heme iron active sites.J Biol Inorg Chem. 2016 Sep;21(5-6):575-88. doi: 10.1007/s00775-016-1372-9. Epub 2016 Jul 1. J Biol Inorg Chem. 2016. PMID: 27369780 Free PMC article. Review.
Cited by
-
Nuclear Resonance Vibrational Spectroscopy Definition of O2 Intermediates in an Extradiol Dioxygenase: Correlation to Crystallography and Reactivity.J Am Chem Soc. 2018 Dec 5;140(48):16495-16513. doi: 10.1021/jacs.8b06517. Epub 2018 Nov 26. J Am Chem Soc. 2018. PMID: 30418018 Free PMC article.
-
β-Hydroxylation of α-amino-β-hydroxylbutanoyl-glycyluridine catalyzed by a nonheme hydroxylase ensures the maturation of caprazamycin.Commun Chem. 2022 Jul 28;5(1):87. doi: 10.1038/s42004-022-00703-6. Commun Chem. 2022. PMID: 36697788 Free PMC article.
-
Nuclear Resonance Vibrational Spectroscopic Definition of the Facial Triad FeIV═O Intermediate in Taurine Dioxygenase: Evaluation of Structural Contributions to Hydrogen Atom Abstraction.J Am Chem Soc. 2020 Nov 4;142(44):18886-18896. doi: 10.1021/jacs.0c08903. Epub 2020 Oct 26. J Am Chem Soc. 2020. PMID: 33103886 Free PMC article.
-
Pangenomic Analysis of Nucleo-Cytoplasmic Large DNA Viruses. I: The Phylogenetic Distribution of Conserved Oxygen-Dependent Enzymes Reveals a Capture-Gene Process.J Mol Evol. 2023 Oct;91(5):647-668. doi: 10.1007/s00239-023-10126-z. Epub 2023 Aug 1. J Mol Evol. 2023. PMID: 37526693 Free PMC article.
-
Dioxygen Activation by Nonheme Diiron Enzymes: Diverse Dioxygen Adducts, High-Valent Intermediates, and Related Model Complexes.Chem Rev. 2018 Mar 14;118(5):2554-2592. doi: 10.1021/acs.chemrev.7b00457. Epub 2018 Feb 5. Chem Rev. 2018. PMID: 29400961 Free PMC article. Review.
References
-
- Solomon EI, Brunold TC, Davis MI, Kemsley JN, Lee SK, Lehnert N, Neese F, Skulan AJ, Yang YS, Zhou J. Geometric and electronic structure/function correlations in non-heme iron enzymes. Chem. Rev. 2000;100:235–349. - PubMed
-
- Falnes PO, Klungland A, Alseth I. Repair of methyl lesions in DNA and RNA by oxidative demethylation. Neuroscience. 2007;145:1222–1232. - PubMed
-
- Baldwin JE, Abraham E. The Biosynthesis of Penicillins and Cephalosporins. Nat. Prod. Reports. 1988;5:129–145. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

