Enzyme Architecture: Self-Assembly of Enzyme and Substrate Pieces of Glycerol-3-Phosphate Dehydrogenase into a Robust Catalyst of Hydride Transfer
- PMID: 27792325
- PMCID: PMC5291162
- DOI: 10.1021/jacs.6b09936
Enzyme Architecture: Self-Assembly of Enzyme and Substrate Pieces of Glycerol-3-Phosphate Dehydrogenase into a Robust Catalyst of Hydride Transfer
Abstract
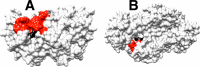
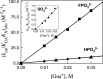
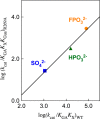
The stabilization of the transition state for hlGPDH-catalyzed reduction of DHAP due to the action of the phosphodianion of DHAP and the cationic side chain of R269 is between 12.4 and 17 kcal/mol. The R269A mutation of glycerol-3-phosphate dehydrogenase (hlGPDH) results in a 9.1 kcal/mol destabilization of the transition state for enzyme-catalyzed reduction of dihydroxyacetone phosphate (DHAP) by NADH, and there is a 6.7 kcal/mol stabilization of this transition state by 1.0 M guanidine cation (Gua+) [J. Am. Chem. Soc. 2015, 137, 5312-5315]. The R269A mutant shows no detectable activity toward reduction of glycolaldehyde (GA), or activation of this reaction by 30 mM HPO32-. We report the unprecedented self-assembly of R269A hlGPDH, dianions (X2- = FPO32-, HPO32-, or SO42-), Gua+ and GA into a functioning catalyst of the reduction of GA, and fourth-order reaction rate constants kcat/KGAKXKGua. The linear logarithmic correlation (slope = 1.0) between values of kcat/KGAKX for dianion activation of wildtype hlGPDH-catalyzed reduction of GA and kcat/KGAKXKGua shows that the electrostatic interaction between exogenous dianions and the side chain of R269 is not significantly perturbed by cutting hlGPDH into R269A and Gua+ pieces. The advantage for connection of hlGPDH (R269A mutant + Gua+) and substrate pieces (GA + HPi) pieces, (ΔGS‡)HPi+E+Gua = 5.6 kcal/mol, is nearly equal to the sum of the advantage to connection of the substrate pieces, (ΔGS‡)GA+HPi = 3.3 kcal/mol, for wildtype hlGPDH-catalyzed reaction of GA + HPi, and for connection of the enzyme pieces, (ΔGS‡)E+Gua = 2.4 kcal/mol, for Gua+ activation of the R269A hlGPDH-catalyzed reaction of DHAP.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




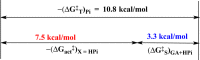
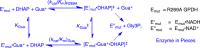



Similar articles
-
Linear Free Energy Relationships for Enzymatic Reactions: Fresh Insight from a Venerable Probe.Acc Chem Res. 2021 May 18;54(10):2532-2542. doi: 10.1021/acs.accounts.1c00147. Epub 2021 May 3. Acc Chem Res. 2021. PMID: 33939414 Free PMC article. Review.
-
Enzyme Architecture: The Role of a Flexible Loop in Activation of Glycerol-3-phosphate Dehydrogenase for Catalysis of Hydride Transfer.Biochemistry. 2018 Jun 12;57(23):3227-3236. doi: 10.1021/acs.biochem.7b01282. Epub 2018 Feb 5. Biochemistry. 2018. PMID: 29337541 Free PMC article.
-
The Organization of Active Site Side Chains of Glycerol-3-phosphate Dehydrogenase Promotes Efficient Enzyme Catalysis and Rescue of Variant Enzymes.Biochemistry. 2020 Apr 28;59(16):1582-1591. doi: 10.1021/acs.biochem.0c00175. Epub 2020 Apr 13. Biochemistry. 2020. PMID: 32250105 Free PMC article.
-
Human Glycerol 3-Phosphate Dehydrogenase: X-ray Crystal Structures That Guide the Interpretation of Mutagenesis Studies.Biochemistry. 2019 Feb 26;58(8):1061-1073. doi: 10.1021/acs.biochem.8b01103. Epub 2019 Jan 31. Biochemistry. 2019. PMID: 30640445 Free PMC article.
-
A reevaluation of the origin of the rate acceleration for enzyme-catalyzed hydride transfer.Org Biomol Chem. 2017 Oct 31;15(42):8856-8866. doi: 10.1039/c7ob01652b. Org Biomol Chem. 2017. PMID: 28956050 Free PMC article. Review.
Cited by
-
Linear Free Energy Relationships for Enzymatic Reactions: Fresh Insight from a Venerable Probe.Acc Chem Res. 2021 May 18;54(10):2532-2542. doi: 10.1021/acs.accounts.1c00147. Epub 2021 May 3. Acc Chem Res. 2021. PMID: 33939414 Free PMC article. Review.
-
Role of the Carboxylate in Enzyme-Catalyzed Decarboxylation of Orotidine 5'-Monophosphate: Transition State Stabilization Dominates Over Ground State Destabilization.J Am Chem Soc. 2019 Aug 28;141(34):13468-13478. doi: 10.1021/jacs.9b04823. Epub 2019 Aug 14. J Am Chem Soc. 2019. PMID: 31365243 Free PMC article.
-
The role of ligand-gated conformational changes in enzyme catalysis.Biochem Soc Trans. 2019 Oct 31;47(5):1449-1460. doi: 10.1042/BST20190298. Biochem Soc Trans. 2019. PMID: 31657438 Free PMC article. Review.
-
Kinetics and mechanism for enzyme-catalyzed reactions of substrate pieces.Methods Enzymol. 2023;685:95-126. doi: 10.1016/bs.mie.2023.03.002. Epub 2023 Apr 18. Methods Enzymol. 2023. PMID: 37245916 Free PMC article.
-
Utilization of Cofactor Binding Energy for Enzyme Catalysis: Formate Dehydrogenase-Catalyzed Reactions of the Whole NAD Cofactor and Cofactor Pieces.Biochemistry. 2023 Aug 1;62(15):2314-2324. doi: 10.1021/acs.biochem.3c00290. Epub 2023 Jul 18. Biochemistry. 2023. PMID: 37463347 Free PMC article.
References
-
- Peracchi A. Curr. Chem. Biol. 2008, 2, 32–49. 10.2174/187231308783334162. - DOI
-
- Reyes A. C.; Koudelka A. P.; Amyes T. L.; Richard J. P. J. Am. Chem. Soc. 2015, 137, 5312–5315. 10.1021/jacs.5b02202. - DOI - PMC - PubMed
- Go M. K.; Amyes T. L.; Richard J. P. J. Am. Chem. Soc. 2010, 132, 13525–13532. 10.1021/ja106104h. - DOI - PMC - PubMed
- Barnett S. A.; Amyes T. L.; Wood M. B.; Gerlt J. A.; Richard J. P. Biochemistry 2010, 49, 824–826. 10.1021/bi902174q. - DOI - PMC - PubMed
- Olucha J.; Meneely K. M.; Lamb A. L. Biochemistry 2012, 51, 7525–7532. 10.1021/bi300472n. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

