Developmental Programming: Insulin Sensitizer Prevents the GnRH-Stimulated LH Hypersecretion in a Sheep Model of PCOS
- PMID: 27792406
- PMCID: PMC5133353
- DOI: 10.1210/en.2016-1613
Developmental Programming: Insulin Sensitizer Prevents the GnRH-Stimulated LH Hypersecretion in a Sheep Model of PCOS
Abstract
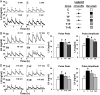
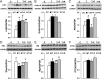
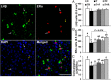
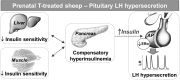
Prenatal testosterone (T) treatment recapitulates the reproductive and metabolic phenotypes of polycystic ovary syndrome in female sheep. At the neuroendocrine level, prenatal T treatment results in disrupted steroid feedback on gonadotropin release, increased pituitary sensitivity to GnRH, and subsequent LH hypersecretion. Because prenatal T-treated sheep manifest functional hyperandrogenism and hyperinsulinemia, gonadal steroids and/or insulin may play a role in programming and/or maintaining these neuroendocrine defects. Here, we investigated the effects of prenatal and postnatal treatments with an androgen antagonist (flutamide [F]) or an insulin sensitizer (rosiglitazone [R]) on GnRH-stimulated LH secretion in prenatal T-treated sheep. As expected, prenatal T treatment increased the pituitary responsiveness to GnRH leading to LH hypersecretion. Neither prenatal interventions nor postnatal F treatment normalized the GnRH-stimulated LH secretion. Conversely, postnatal R treatment completely normalized the GnRH-stimulated LH secretion. At the tissue level, gestational T increased pituitary LHβ, androgen receptor, and insulin receptor-β, whereas it reduced estrogen receptor (ER)α protein levels. Although postnatal F normalized pituitary androgen receptor and insulin receptor-β, it failed to prevent an increase in LHβ expression. Contrarily, postnatal R treatment restored ERα and partially normalized LHβ pituitary levels. Immunohistochemical findings confirmed changes in pituitary ERα expression to be specific to gonadotropes. In conclusion, these findings indicate that increased pituitary responsiveness to GnRH in prenatal T-treated sheep is likely a function of reduced peripheral insulin sensitivity. Moreover, results suggest that restoration of ERα levels in the pituitary may be one mechanism by which R prevents GnRH-stimulated LH hypersecretion in this sheep model of polycystic ovary syndrome-like phenotype.
Figures


Similar articles
-
Developmental programming: gestational testosterone excess disrupts LH secretion in the female sheep fetus.Reprod Biol Endocrinol. 2020 Nov 7;18(1):106. doi: 10.1186/s12958-020-00667-z. Reprod Biol Endocrinol. 2020. PMID: 33158439 Free PMC article.
-
Developmental Programming: Prenatal and Postnatal Androgen Antagonist and Insulin Sensitizer Interventions Prevent Advancement of Puberty and Improve LH Surge Dynamics in Prenatal Testosterone-Treated Sheep.Endocrinology. 2015 Jul;156(7):2678-92. doi: 10.1210/en.2015-1235. Epub 2015 Apr 28. Endocrinology. 2015. PMID: 25919188 Free PMC article.
-
Developmental programming: prenatal and postnatal contribution of androgens and insulin in the reprogramming of estradiol positive feedback disruptions in prenatal testosterone-treated sheep.Endocrinology. 2012 Jun;153(6):2813-22. doi: 10.1210/en.2011-2074. Epub 2012 Mar 27. Endocrinology. 2012. PMID: 22454153 Free PMC article.
-
Developmental programming of the neuroendocrine axis by steroid hormones: Insights from the sheep model of PCOS.Front Endocrinol (Lausanne). 2023 Jan 23;14:1096187. doi: 10.3389/fendo.2023.1096187. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 36755919 Free PMC article. Review.
-
Steroidogenic versus Metabolic Programming of Reproductive Neuroendocrine, Ovarian and Metabolic Dysfunctions.Neuroendocrinology. 2015;102(3):226-37. doi: 10.1159/000381830. Epub 2015 Apr 1. Neuroendocrinology. 2015. PMID: 25832114 Free PMC article. Review.
Cited by
-
Erythropoietin-producing hepatocellular A7 triggering ovulation indicates a potential beneficial role for polycystic ovary syndrome.EBioMedicine. 2018 Oct;36:539-552. doi: 10.1016/j.ebiom.2018.09.046. Epub 2018 Oct 3. EBioMedicine. 2018. PMID: 30292674 Free PMC article.
-
Ovarian and Extra-Ovarian Mediators in the Development of Polycystic Ovary Syndrome.J Mol Endocrinol. 2018 Oct 16;61(4):R161-R184. doi: 10.1530/JME-18-0079. J Mol Endocrinol. 2018. PMID: 29941488 Free PMC article. Review.
-
Nonhuman Primates: A Vital Model for Basic and Applied Research on Female Reproduction, Prenatal Development, and Women's Health.ILAR J. 2017 Dec 1;58(2):281-294. doi: 10.1093/ilar/ilx027. ILAR J. 2017. PMID: 28985318 Free PMC article. Review.
-
Animal Models to Understand the Etiology and Pathophysiology of Polycystic Ovary Syndrome.Endocr Rev. 2020 Jul 1;41(4):bnaa010. doi: 10.1210/endrev/bnaa010. Endocr Rev. 2020. PMID: 32310267 Free PMC article. Review.
-
Letrozole Rat Model Mimics Human Polycystic Ovarian Syndrome and Changes in Insulin Signal Pathways.Med Sci Monit. 2020 Jul 8;26:e923073. doi: 10.12659/MSM.923073. Med Sci Monit. 2020. PMID: 32638705 Free PMC article.
References
-
- Homburg R. Polycystic ovary syndrome - from gynaecological curiosity to multisystem endocrinopathy. Hum Reprod. 1996;11(1):29–39. - PubMed
-
- Kousta E, White DM, Cela E, McCarthy MI, Franks S. The prevalence of polycystic ovaries in women with infertility. Hum Reprod. 1999;14(11):2720–2723. - PubMed
-
- Balen A, Michelmore K. What is polycystic ovary syndrome? Are national views important? Hum Reprod. 2002;17(9):2219–2227. - PubMed
-
- Eagleson CA, Gingrich MB, Pastor CL, et al. Polycystic ovarian syndrome: evidence that flutamide restores sensitivity of the gonadotropin-releasing hormone pulse generator to inhibition by estradiol and progesterone 1. J Clin Endocrinol Metab. 2000;85(11):4047–4052. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

