STING-Licensed Macrophages Prime Type I IFN Production by Plasmacytoid Dendritic Cells in the Bone Marrow during Severe Plasmodium yoelii Malaria
- PMID: 27792766
- PMCID: PMC5085251
- DOI: 10.1371/journal.ppat.1005975
STING-Licensed Macrophages Prime Type I IFN Production by Plasmacytoid Dendritic Cells in the Bone Marrow during Severe Plasmodium yoelii Malaria
Abstract
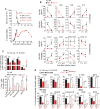
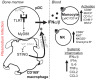
Malaria remains a global health burden causing significant morbidity, yet the mechanisms underlying disease outcomes and protection are poorly understood. Herein, we analyzed the peripheral blood of a unique cohort of Malawian children with severe malaria, and performed a comprehensive overview of blood leukocytes and inflammatory mediators present in these patients. We reveal robust immune cell activation, notably of CD14+ inflammatory monocytes, NK cells and plasmacytoid dendritic cells (pDCs) that is associated with very high inflammation. Using the Plasmodium yoelii 17X YM surrogate mouse model of lethal malaria, we report a comparable pattern of immune cell activation and inflammation and found that type I IFN represents a key checkpoint for disease outcomes. Compared to wild type mice, mice lacking the type I interferon (IFN) receptor exhibited a significant decrease in immune cell activation and inflammatory response, ultimately surviving the infection. We demonstrate that pDCs were the major producers of systemic type I IFN in the bone marrow and the blood of infected mice, via TLR7/MyD88-mediated recognition of Plasmodium parasites. This robust type I IFN production required priming of pDCs by CD169+ macrophages undergoing activation upon STING-mediated sensing of parasites in the bone marrow. pDCs and macrophages displayed prolonged interactions in this compartment in infected mice as visualized by intravital microscopy. Altogether our findings describe a novel mechanism of pDC activation in vivo and precise stepwise cell/cell interactions taking place during severe malaria that contribute to immune cell activation and inflammation, and subsequent disease outcomes.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures






References
-
- Crompton PD, Moebius J, Portugal S, Waisberg M, Hart G, Garver LS, et al. Malaria immunity in man and mosquito: insights into unsolved mysteries of a deadly infectious disease. Annu Rev Immunol. 2014;32:157–87. PubMed Central PMCID: PMC4075043. 10.1146/annurev-immunol-032713-120220 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

