Isoniazid clearance is impaired among human immunodeficiency virus/tuberculosis patients with high levels of immune activation
- PMID: 27792837
- PMCID: PMC5346858
- DOI: 10.1111/bcp.13172
Isoniazid clearance is impaired among human immunodeficiency virus/tuberculosis patients with high levels of immune activation
Abstract
Aims: Immune activation, which is characteristic of both tuberculosis (TB) and human immunodeficiency virus (HIV) infection, is associated with impaired drug metabolism. We tested the hypothesis that elevated levels of systemic immune activation among adults with HIV/TB initiating antiretroviral therapy (ART) would be associated with impaired clearance of isoniazid.
Methods: We conducted a prospective observational study of isoniazid pharmacokinetics (PK) and systemic immune activation prior to and 1 month after ART initiation. Nonlinear mixed effects analysis was performed to measure the covariate effect of immune activation on isoniazid clearance in a model that also included N-acetyltransferase-2 (NAT-2) genotype and interoccasional variability on clearance (thereby analyzing the PK data before and after ART initiation in a single model).
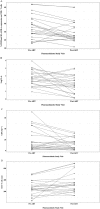
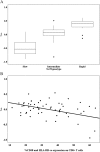
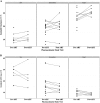
Results: We enrolled 40 patients in the PK visit prior to ART, and 24 patients returned for the second visit a median of 33 days after initiating antiretroviral therapy. The isoniazid concentration data were best described by a two-compartment model with first-order elimination. After accounting for NAT-2 genotype, increasing levels of CD38 and HLA-DR expression on CD8+ T cells (CD38+ DR+ CD8+ ) were associated with decreasing isoniazid clearance.
Conclusion: HIV/TB patients with high levels of immune activation demonstrated impaired isoniazid clearance. Future efforts should determine the role of this relationship in clinical hepatotoxicity events.
Keywords: human immunodeficiency virus; immune activation; isoniazid; n-acetyltransferse-2; pharmacokinetics; tuberculosis.
© 2016 The British Pharmacological Society.
Figures
References
-
- Forget EJ, Menzies D. Adverse reactions to first‐line antituberculosis drugs. Expert Opin Drug Saf 2006; 5: 231–249. - PubMed
-
- Lee WM. Drug‐induced hepatotoxicity. N Engl J Med 2003; 349: 474–485. - PubMed
-
- Preziosi P. Isoniazid: metabolic aspects and toxicological correlates. Curr Drug Metab 2007; 8: 839–851. - PubMed
-
- Metushi IG, Cai P, Zhu X, Nakagawa T, Uetrecht JP. A fresh look at the mechanism of isoniazid‐induced hepatotoxicity. Clin Pharmacol Ther 2011; 89: 911–914. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

