Methylation-associated silencing of microRNA-129-3p promotes epithelial-mesenchymal transition, invasion and metastasis of hepatocelluar cancer by targeting Aurora-A
- PMID: 27793005
- PMCID: PMC5363640
- DOI: 10.18632/oncotarget.12870
Methylation-associated silencing of microRNA-129-3p promotes epithelial-mesenchymal transition, invasion and metastasis of hepatocelluar cancer by targeting Aurora-A
Abstract
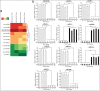
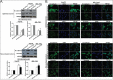
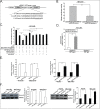
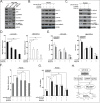
Metastasis and recurrence has become one major obstacle for further improving the survival of hepatocelluar cancer (HCC) patients. Therefore, it is critical to elucidate the mechanisms involved in HCC metastasis. This study aimed to investigate the roles of microRNA (miR)-129-3p in HCC metastasis and its possible molecular mechanisms. By using microarray analysis to compare levels of different miRNAs in HCC tissues with or without lymph node metastasis (LNM), we showed that HCC tissues with LNM had reduced levels of miR-129-3p, which was related to its promoter hypermethylation and correlated with tumor metastasis, recurrence and poor prognosis. Gain - and loss - of - function assays indicated that re-expression of miR-129-3p could reverse epithelial-mesenchymal transition (EMT), and reduce in vitro invasion and in vivo metastasis of HCC cells. Aurora-A, a serine/threonine protein kinase, was identified as a direct target of miR-129-3p. Knockdown of Aurora-A phenocopied the effect of miR-129-3p overexpression on HCC metastasis. In addition, Aurora-A upregulation could partially rescue the effect of miR-129-3p. We further demonstrated that activation of PI3K/Akt and p38-MAPK signalings were involved in miR-129-3p-mediated HCC metastasis. These findings suggest that methylation-mediated miR-129-3p downregulation promotes EMT, in vitro invasion and in vivo metastasis of HCC cells via activation of PI3K/Akt and p38-MAPK signalings partially by targeting Aurora-A. Therefore, miR-129-3p may be a novel prognostic biomarker and potential therapeutic target for HCC.
Keywords: Aurora-A; epithelial-mesenchymal transition; hepatocelluar cancer; metastasis; miR-129-3p.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures





References
-
- Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. - PubMed
-
- Zhou XD. Recurrence and metastasis of hepatocellular carcinoma: progress and prospects. Hepatobiliary Pancreat Dis Int. 2002;1:35–41. - PubMed
-
- Chen PY, Meister G. microRNA-guided posttranscriptional gene regulation. Biol Chem. 2005;386:1205–1218. - PubMed
-
- Li C, Hashimi SM, Good DA, Cao S, Duan W, Plummer PN, Mellick AS, Wei MQ. Apoptosis and microRNA aberrations in cancer. Clin Exp Pharmacol Physiol. 2012;39:739–746. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

