Absence of ERK5/MAPK7 delays tumorigenesis in Atm-/- mice
- PMID: 27793024
- PMCID: PMC5342677
- DOI: 10.18632/oncotarget.12908
Absence of ERK5/MAPK7 delays tumorigenesis in Atm-/- mice
Abstract
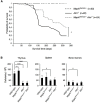
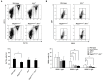
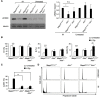
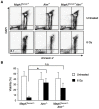
Ataxia-telangiectasia mutated (ATM) is a cell cycle checkpoint kinase that upon activation by DNA damage leads to cell cycle arrest and DNA repair or apoptosis. The absence of Atm or the occurrence of loss-of-function mutations in Atm predisposes to tumorigenesis. MAPK7 has been implicated in numerous types of cancer with pro-survival and pro-growth roles in tumor cells, but its functional relation with tumor suppressors is not clear. In this study, we show that absence of MAPK7 delays death due to spontaneous tumor development in Atm-/- mice. Compared with Atm-/- thymocytes, Mapk7-/-Atm-/- thymocytes exhibited an improved response to DNA damage (increased phosphorylation of H2AX) and a restored apoptotic response after treatment of mice with ionizing radiation. These findings define an antagonistic function of ATM and MAPK7 in the thymocyte response to DNA damage, and suggest that the lack of MAPK7 inhibits thymic lymphoma growth in Atm-/- mice by partially restoring the DNA damage response in thymocytes.
Keywords: BMK1; DNA damage response; thymic lymphoma; thymocyte; γH2AX.
Conflict of interest statement
No potential conflicts of interest were disclosed.
Figures


Comment in
-
ATM/MAPK7 crosstalk in cancer.Oncotarget. 2017 Feb 28;8(9):14271-14272. doi: 10.18632/oncotarget.15367. Oncotarget. 2017. PMID: 28212585 Free PMC article. No abstract available.
References
-
- Guo Z, Kozlov S, Lavin MF, Person MD, Paull TT. ATM activation by oxidative stress. Science. 2010;330:517–521. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

