Cranial irradiation induces transient microglia accumulation, followed by long-lasting inflammation and loss of microglia
- PMID: 27793054
- PMCID: PMC5347693
- DOI: 10.18632/oncotarget.12929
Cranial irradiation induces transient microglia accumulation, followed by long-lasting inflammation and loss of microglia
Abstract
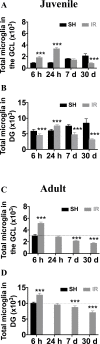
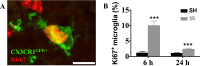
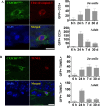
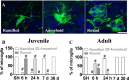
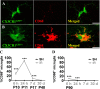
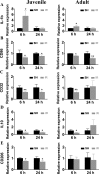
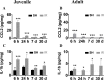
The relative contribution of resident microglia and peripheral monocyte-derived macrophages in neuroinflammation after cranial irradiation is not known. A single dose of 8 Gy was administered to postnatal day 10 (juvenile) or 90 (adult) CX3CR1GFP/+ CCR2RFP/+ mouse brains. Microglia accumulated in the subgranular zone of the hippocampal granule cell layer, where progenitor cell death was prominent. The peak was earlier (6 h vs. 24 h) but less pronounced in adult brains. The increase in juvenile, but not adult, brains was partly attributed to proliferation. Microglia numbers then decreased over time to 39% (juvenile) and 58% (adult) of controls 30 days after irradiation, largely as a result of cell death. CD68 was expressed in 90% of amoeboid microglia in juvenile hippocampi but only in 9% of adult ones. Isolated hippocampal microglia revealed reduced CD206 and increased IL1-beta expression after irradiation, more pronounced in juvenile brains. CCL2 and IL-1 beta increased after irradiation, more in juvenile hippocampi, and remained elevated at all time points. In summary, microglia activation after irradiation was more pronounced, protracted and pro-inflammatory by nature in juvenile than in adult hippocampi. Common to both ages was long-lasting inflammation and the absence of monocyte-derived macrophages.
Keywords: irradiation; macrophage; microglia; monocyte; neurogenesis; neuroinflammation.
Conflict of interest statement
The author(s) declare that they have no competing interests.
Figures

Similar articles
-
Lipopolysaccharide-induced inflammation aggravates irradiation-induced injury to the young mouse brain.Dev Neurosci. 2013;35(5):406-15. doi: 10.1159/000353820. Epub 2013 Aug 20. Dev Neurosci. 2013. PMID: 23970040
-
Different reactions to irradiation in the juvenile and adult hippocampus.Int J Radiat Biol. 2014 Sep;90(9):807-15. doi: 10.3109/09553002.2014.942015. Epub 2014 Aug 11. Int J Radiat Biol. 2014. PMID: 25004947
-
Microglia depletion and repopulation do not alter the effects of cranial irradiation on hippocampal neurogenesis.Brain Behav Immun. 2025 Jan;123:57-63. doi: 10.1016/j.bbi.2024.08.055. Epub 2024 Aug 30. Brain Behav Immun. 2025. PMID: 39218233
-
Pathological Mechanisms of Irradiation-Induced Neurological Deficits in the Developing Brain.Eur J Neurosci. 2025 Mar;61(6):e70070. doi: 10.1111/ejn.70070. Eur J Neurosci. 2025. PMID: 40098303 Review.
-
Microglia as Therapeutic Target for Radiation-Induced Brain Injury.Int J Mol Sci. 2022 Jul 27;23(15):8286. doi: 10.3390/ijms23158286. Int J Mol Sci. 2022. PMID: 35955439 Free PMC article. Review.
Cited by
-
Metformin pretreatment rescues olfactory memory associated with subependymal zone neurogenesis in a juvenile model of cranial irradiation.Cell Rep Med. 2021 Apr 6;2(4):100231. doi: 10.1016/j.xcrm.2021.100231. eCollection 2021 Apr 20. Cell Rep Med. 2021. PMID: 33948569 Free PMC article.
-
Radiation induces progenitor cell death, microglia activation, and blood-brain barrier damage in the juvenile rat cerebellum.Sci Rep. 2017 Apr 6;7:46181. doi: 10.1038/srep46181. Sci Rep. 2017. PMID: 28382975 Free PMC article.
-
Immune landscape of isocitrate dehydrogenase-stratified primary and recurrent human gliomas.Neuro Oncol. 2024 Dec 5;26(12):2239-2255. doi: 10.1093/neuonc/noae139. Neuro Oncol. 2024. PMID: 39126294 Free PMC article.
-
Mitochondrial Translocator Protein (TSPO) Expression in the Brain After Whole Body Gamma Irradiation.Front Cell Dev Biol. 2021 Oct 25;9:715444. doi: 10.3389/fcell.2021.715444. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34760884 Free PMC article.
-
Microglial dynamics and emerging therapeutic strategies in CNS homeostasis and pathology.Front Pharmacol. 2025 May 13;16:1577809. doi: 10.3389/fphar.2025.1577809. eCollection 2025. Front Pharmacol. 2025. PMID: 40432891 Free PMC article. Review.
References
-
- Mulhern RK, Merchant TE, Gajjar A, Reddick WE, Kun LE. Late neurocognitive sequelae in survivors of brain tumours in childhood. Lancet Oncol. 2004;5:399–408. - PubMed
-
- Packer RJ, Sutton LN, Atkins TE, Radcliffe J, Bunin GR, D'Angio G, Siegel KR, Schut L. A prospective study of cognitive function in children receiving whole-brain radiotherapy and chemotherapy: 2-year results. J Neurosurg. 1989;70:707–13. - PubMed
-
- Crossen JR, Garwood D, Glatstein E, Neuwelt EA. Neurobehavioral sequelae of cranial irradiation in adults: a review of radiation-induced encephalopathy. J Clin Oncol. 1994;12:627–42. - PubMed
-
- Raber J, Rola R, LeFevour A, Morhardt D, Curley J, Mizumatsu S, VandenBerg SR, Fike JR. Radiation-induced cognitive impairments are associated with changes in indicators of hippocampal neurogenesis. Radiat Res. 2004;162:39–47. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

