MOG-IgG in NMO and related disorders: a multicenter study of 50 patients. Part 2: Epidemiology, clinical presentation, radiological and laboratory features, treatment responses, and long-term outcome
- PMID: 27793206
- PMCID: PMC5086042
- DOI: 10.1186/s12974-016-0718-0
MOG-IgG in NMO and related disorders: a multicenter study of 50 patients. Part 2: Epidemiology, clinical presentation, radiological and laboratory features, treatment responses, and long-term outcome
Abstract
Background: A subset of patients with neuromyelitis optica spectrum disorders (NMOSD) has been shown to be seropositive for myelin oligodendrocyte glycoprotein antibodies (MOG-IgG).
Objective: To describe the epidemiological, clinical, radiological, cerebrospinal fluid (CSF), and electrophysiological features of a large cohort of MOG-IgG-positive patients with optic neuritis (ON) and/or myelitis (n = 50) as well as attack and long-term treatment outcomes.
Methods: Retrospective multicenter study.
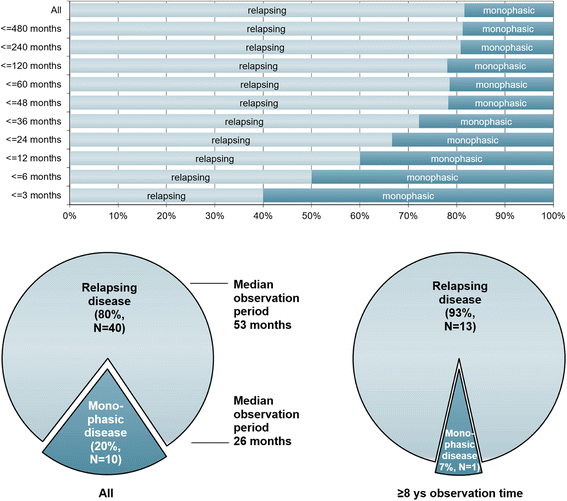
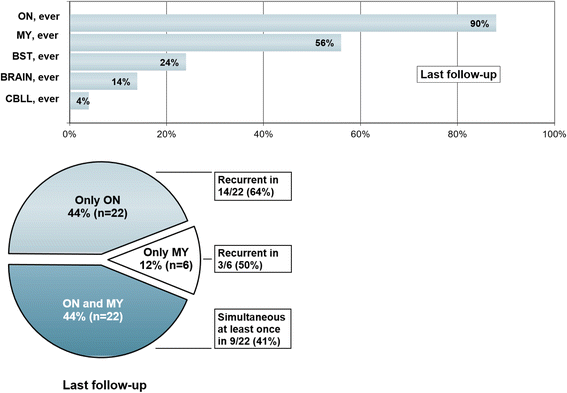
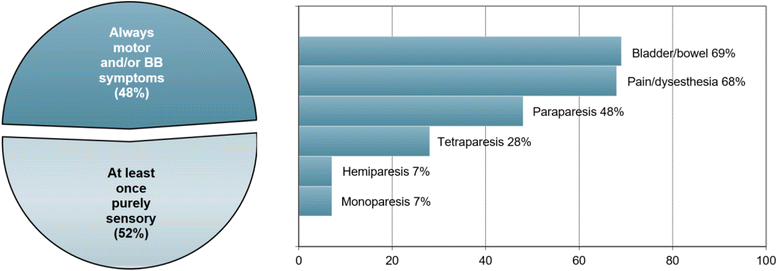
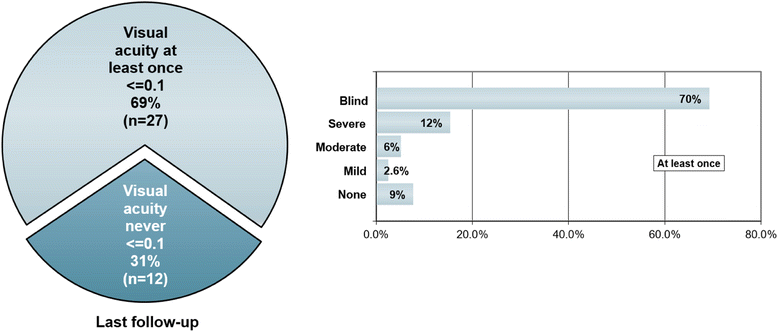
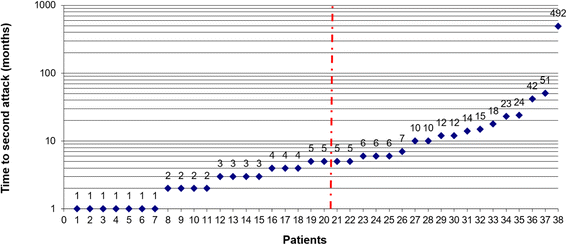
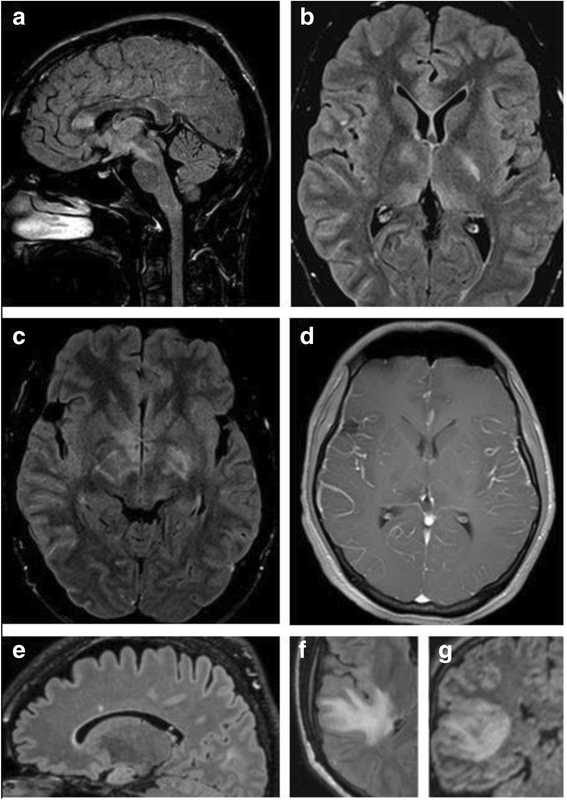
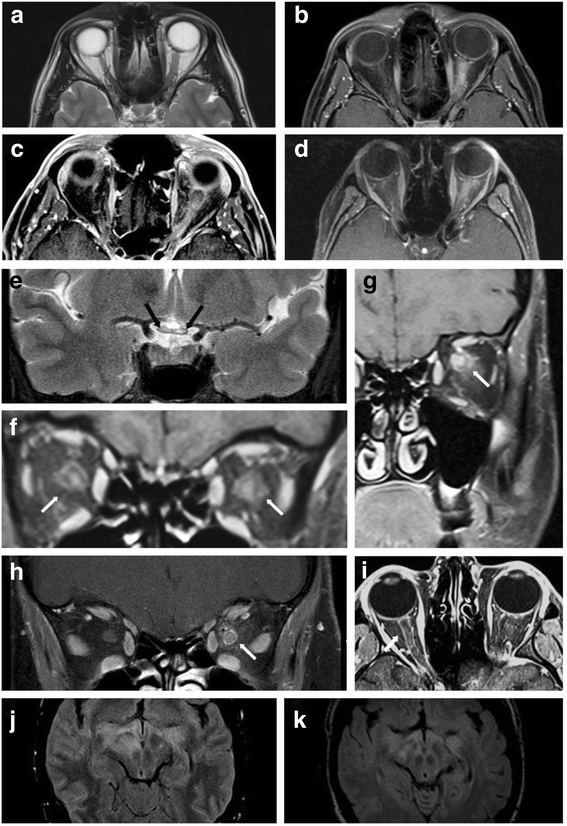
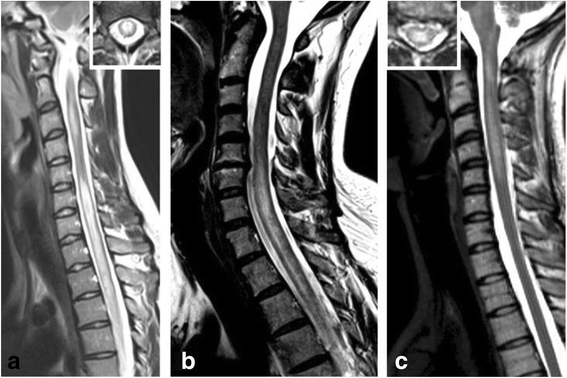
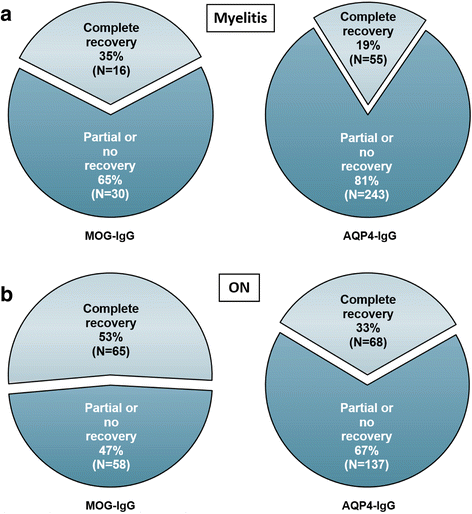
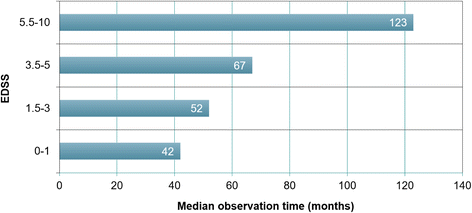
Results: The sex ratio was 1:2.8 (m:f). Median age at onset was 31 years (range 6-70). The disease followed a multiphasic course in 80 % (median time-to-first-relapse 5 months; annualized relapse rate 0.92) and resulted in significant disability in 40 % (mean follow-up 75 ± 46.5 months), with severe visual impairment or functional blindness (36 %) and markedly impaired ambulation due to paresis or ataxia (25 %) as the most common long-term sequelae. Functional blindess in one or both eyes was noted during at least one ON attack in around 70 %. Perioptic enhancement was present in several patients. Besides acute tetra-/paraparesis, dysesthesia and pain were common in acute myelitis (70 %). Longitudinally extensive spinal cord lesions were frequent, but short lesions occurred at least once in 44 %. Fourty-one percent had a history of simultaneous ON and myelitis. Clinical or radiological involvement of the brain, brainstem, or cerebellum was present in 50 %; extra-opticospinal symptoms included intractable nausea and vomiting and respiratory insufficiency (fatal in one). CSF pleocytosis (partly neutrophilic) was present in 70 %, oligoclonal bands in only 13 %, and blood-CSF-barrier dysfunction in 32 %. Intravenous methylprednisolone (IVMP) and long-term immunosuppression were often effective; however, treatment failure leading to rapid accumulation of disability was noted in many patients as well as flare-ups after steroid withdrawal. Full recovery was achieved by plasma exchange in some cases, including after IVMP failure. Breakthrough attacks under azathioprine were linked to the drug-specific latency period and a lack of cotreatment with oral steroids. Methotrexate was effective in 5/6 patients. Interferon-beta was associated with ongoing or increasing disease activity. Rituximab and ofatumumab were effective in some patients. However, treatment with rituximab was followed by early relapses in several cases; end-of-dose relapses occurred 9-12 months after the first infusion. Coexisting autoimmunity was rare (9 %). Wingerchuk's 2006 and 2015 criteria for NMO(SD) and Barkhof and McDonald criteria for multiple sclerosis (MS) were met by 28 %, 32 %, 15 %, 33 %, respectively; MS had been suspected in 36 %. Disease onset or relapses were preceded by infection, vaccination, or pregnancy/delivery in several cases.
Conclusion: Our findings from a predominantly Caucasian cohort strongly argue against the concept of MOG-IgG denoting a mild and usually monophasic variant of NMOSD. The predominantly relapsing and often severe disease course and the short median time to second attack support the use of prophylactic long-term treatments in patients with MOG-IgG-positive ON and/or myelitis.
Keywords: Aquaporin-4 antibodies (AQP4-IgG, NMO-IgG); Autoantibodies; Azathioprine; Barkhof criteria; Cerebrospinal fluid; Electrophysiology; Evoked potentials; Glatiramer acetate; IPND criteria; Infections; Interferon beta; International consensus diagnostic criteria for neuromyelitis optica spectrum disorders; Longitudinally extensive transverse myelitis; Magnetic resonance imaging; McDonald criteria; Methotrexate; Multiple sclerosis; Myelin oligodendrocyte glycoprotein antibodies (MOG-IgG); Natalizumab; Neuromyelitis optica spectrum disorders (NMOSD); Ofatumumab; Oligoclonal bands; Optic neuritis; Outcome; Pregnancy; Rituximab; Therapy; Transverse myelitis; Treatment; Vaccination; Wingerchuk criteria 2006 and 2015.
Figures


References
-
- Jarius S, Paul F, Franciotta D, Waters P, Zipp F, Hohlfeld R, Vincent A, Wildemann B. Mechanisms of disease: aquaporin-4 antibodies in neuromyelitis optica. Nat. Clin. Pract. Neurol. 2008;4:202–14. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

