Dendritic Cells and Cancer Immunity
- PMID: 27793569
- PMCID: PMC5135568
- DOI: 10.1016/j.it.2016.09.006
Dendritic Cells and Cancer Immunity
Abstract
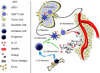
Dendritic cells (DCs) are central regulators of the adaptive immune response, and as such are necessary for T-cell-mediated cancer immunity. In particular, antitumoral responses depend on a specialized subset of conventional DCs that transport tumor antigens to draining lymph nodes and cross-present antigen to activate cytotoxic T lymphocytes. DC maturation is necessary to provide costimulatory signals to T cells, but while DC maturation occurs within tumors, it is often insufficient to induce potent immunity, particularly in light of suppressive mechanisms within tumors. Bypassing suppressive pathways or directly activating DCs can unleash a T-cell response, and although clinical efficacy has proven elusive, therapeutic targeting of DCs continues to hold translational potential in combinatorial approaches.
Keywords: antigen presentation; cancer; dendritic cells; immunotherapy; tumor microenvironment; vaccination.
Copyright © 2016 Elsevier Ltd. All rights reserved.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

