Immunomodulatory Functions of BTLA and HVEM Govern Induction of Extrathymic Regulatory T Cells and Tolerance by Dendritic Cells
- PMID: 27793593
- PMCID: PMC5112132
- DOI: 10.1016/j.immuni.2016.10.008
Immunomodulatory Functions of BTLA and HVEM Govern Induction of Extrathymic Regulatory T Cells and Tolerance by Dendritic Cells
Abstract
Dendritic cells (DCs) initiate immunity and also antigen-specific tolerance mediated by extrathymic regulatory T (Treg) cells, yet it remains unclear how DCs regulate induction of such tolerance. Here, we report that efficient induction of Treg cells was instructed by BTLA+DEC205+CD8+CD11c+ DCs and the immunomodulatory functions of BTLA. In contrast, T cell activation in steady state by total CD11c+ DCs that include a majority of DCs that do not express BTLA did not induce Treg cells and had no lasting impact on subsequent immune responses. Engagement of HVEM, a receptor of BTLA, promoted Foxp3 expression in T cells through upregulation of CD5. In contrast, T cells activated in the absence of BTLA and HVEM-mediated functions remained CD5lo and therefore failed to resist the inhibition of Foxp3 expression in response to effector cell-differentiating cytokines. Thus, DCs require BTLA and CD5-dependent mechanisms to actively adjust tolerizing T cell responses under steady-state conditions.
Copyright © 2016 Elsevier Inc. All rights reserved.
Figures
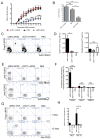

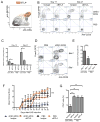
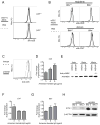
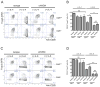
Comment in
-
BTLA+ Dendritic Cells: The Regulatory T Cell Force Awakens.Immunity. 2016 Nov 15;45(5):956-958. doi: 10.1016/j.immuni.2016.10.030. Immunity. 2016. PMID: 27851922
References
-
- Albring JC, Sandau MM, Rapaport AS, Edelson BT, Satpathy A, Mashayekhi M, Lathrop SK, Hsieh CS, Stelljes M, Colonna M, et al. Targeting of B and T lymphocyte associated (BTLA) prevents graft-versus-host disease without global immunosuppression. The Journal of experimental medicine. 2010;207:2551–2559. - PMC - PubMed
-
- Azzam HS, DeJarnette JB, Huang K, Emmons R, Park CS, Sommers CL, El-Khoury D, Shores EW, Love PE. Fine tuning of TCR signaling by CD5. Journal of immunology. 2001;166:5464–5472. - PubMed
-
- Barnden MJ, Allison J, Heath WR, Carbone FR. Defective TCR expression in transgenic mice constructed using cDNA-based alpha- and beta-chain genes under the control of heterologous regulatory elements. Immunol Cell Biol. 1998;76:34–40. - PubMed
-
- Belz GT, Behrens GM, Smith CM, Miller JF, Jones C, Lejon K, Fathman CG, Mueller SN, Shortman K, Carbone FR, Heath WR. The CD8alpha(+) dendritic cell is responsible for inducing peripheral self-tolerance to tissue-associated antigens. The Journal of experimental medicine. 2002;196:1099–1104. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

