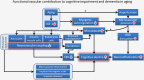
Functional vascular contributions to cognitive impairment and dementia: mechanisms and consequences of cerebral autoregulatory dysfunction, endothelial impairment, and neurovascular uncoupling in aging
- PMID: 27793855
- PMCID: PMC5283909
- DOI: 10.1152/ajpheart.00581.2016
Functional vascular contributions to cognitive impairment and dementia: mechanisms and consequences of cerebral autoregulatory dysfunction, endothelial impairment, and neurovascular uncoupling in aging
Abstract
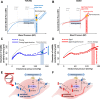
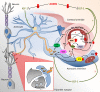
Increasing evidence from epidemiological, clinical and experimental studies indicate that age-related cerebromicrovascular dysfunction and microcirculatory damage play critical roles in the pathogenesis of many types of dementia in the elderly, including Alzheimer's disease. Understanding and targeting the age-related pathophysiological mechanisms that underlie vascular contributions to cognitive impairment and dementia (VCID) are expected to have a major role in preserving brain health in older individuals. Maintenance of cerebral perfusion, protecting the microcirculation from high pressure-induced damage and moment-to-moment adjustment of regional oxygen and nutrient supply to changes in demand are prerequisites for the prevention of cerebral ischemia and neuronal dysfunction. This overview discusses age-related alterations in three main regulatory paradigms involved in the regulation of cerebral blood flow (CBF): cerebral autoregulation/myogenic constriction, endothelium-dependent vasomotor function, and neurovascular coupling responses responsible for functional hyperemia. The pathophysiological consequences of cerebral microvascular dysregulation in aging are explored, including blood-brain barrier disruption, neuroinflammation, exacerbation of neurodegeneration, development of cerebral microhemorrhages, microvascular rarefaction, and ischemic neuronal dysfunction and damage. Due to the widespread attention that VCID has captured in recent years, the evidence for the causal role of cerebral microvascular dysregulation in cognitive decline is critically examined.
Keywords: Alzheimer’s disease; blood-brain barrier; cerebral circulation; cerebrovascular; functional hyperemia; geroscience; microcirculation; myogenic constriction; neurovascular coupling; senescence; stroke; vascular aging.
Copyright © 2017 the American Physiological Society.
Figures

References
-
- Abellan van Kan G, Rolland Y, Andrieu S, Bauer J, Beauchet O, Bonnefoy M, Cesari M, Donini LM, Gillette Guyonnet S, Inzitari M, Nourhashemi F, Onder G, Ritz P, Salva A, Visser M, Vellas B. Gait speed at usual pace as a predictor of adverse outcomes in community-dwelling older people an International Academy on Nutrition and Aging (IANA) Task Force. J Nutr Health Aging 13: 881–889, 2009. doi:10.1007/s12603-009-0246-z. - DOI - PubMed
-
- Angelini A, Bendini C, Neviani F, Bergamini L, Manni B, Trenti T, Rovati R, Neri M. Insulin-like growth factor-1 (IGF-1): relation with cognitive functioning and neuroimaging marker of brain damage in a sample of hypertensive elderly subjects. Arch Gerontol Geriatr 49, Suppl 1: 5–12, 2009. doi:10.1016/j.archger.2009.09.006. - DOI - PubMed
-
- Aukes AM, Vitullo L, Zeeman GG, Cipolla MJ. Pregnancy prevents hypertensive remodeling and decreases myogenic reactivity in posterior cerebral arteries from Dahl salt-sensitive rats: a role in eclampsia? Am J Physiol Heart Circ Physiol 292: H1071–H1076, 2007. doi:10.1152/ajpheart.00980.2006. - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

