The Role of Sialylated Glycans in Human Platelet Endothelial Cell Adhesion Molecule 1 (PECAM-1)-mediated Trans Homophilic Interactions and Endothelial Cell Barrier Function
- PMID: 27793989
- PMCID: PMC5207088
- DOI: 10.1074/jbc.M116.756502
The Role of Sialylated Glycans in Human Platelet Endothelial Cell Adhesion Molecule 1 (PECAM-1)-mediated Trans Homophilic Interactions and Endothelial Cell Barrier Function
Abstract
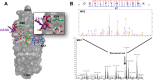
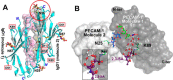
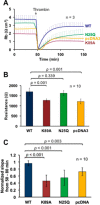
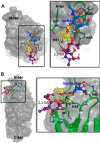
Platelet Endothelial Cell Adhesion Molecule 1 (PECAM-1) is a major component of the endothelial cell intercellular junction. Previous studies have shown that PECAM-1 homophilic interactions, mediated by amino-terminal immunoglobulin homology domain 1, contribute to maintenance of the vascular permeability barrier and to its re-establishment following inflammatory or thrombotic insult. PECAM-1 glycans account for ∼30% of its molecular mass, and the newly solved crystal structure of human PECAM-1 immunoglobulin homology domain 1 reveals that a glycan emanating from the asparagine residue at position 25 (Asn-25) is located within the trans homophilic-binding interface, suggesting a role for an Asn-25-associated glycan in PECAM-1 homophilic interactions. In support of this possibility, unbiased molecular docking studies revealed that negatively charged α2,3 sialic acid moieties bind tightly to a groove within the PECAM-1 homophilic interface in an orientation that favors the formation of an electrostatic bridge with positively charged Lys-89, mutation of which has been shown previously to disrupt PECAM-1-mediated homophilic binding. To verify the contribution of the Asn-25 glycan to endothelial barrier function, we generated an N25Q mutant form of PECAM-1 that is not glycosylated at this position and examined its ability to contribute to vascular integrity in endothelial cell-like REN cells. Confocal microscopy showed that although N25Q PECAM-1 concentrates normally at cell-cell junctions, the ability of this mutant form of PECAM-1 to support re-establishment of a permeability barrier following disruption with thrombin was significantly compromised. Taken together, these data suggest that a sialic acid-containing glycan emanating from Asn-25 reinforces dynamic endothelial cell-cell interactions by stabilizing the PECAM-1 homophilic binding interface.
Keywords: adhesion; endothelial cell; glycosylation; permeability; platelet endothelial cell adhesion molecule (PECAM); sialic acid; vascular biology.
© 2016 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures


References
-
- Newman P. J., Berndt M. C., Gorski J., White G. C. 2nd, Lyman S., Paddock C., and Muller W. A. (1990) PECAM-1 (CD31) cloning and relation to adhesion molecules of the immunoglobulin gene superfamily. Science 247, 1219–1222 - PubMed
-
- Newman P. J., and Newman D. K. (2003) Signal transduction pathways mediated by PECAM-1: new roles for an old molecule in platelet and vascular cell biology. Arterioscler. Thromb. Vasc. Biol. 23, 953–964 - PubMed
-
- Sun Q.-H., DeLisser H. M., Zukowski M. M., Paddock C., Albelda S. M., and Newman P. J. (1996) Individually distinct Ig homology domains in PECAM-1 regulate homophilic binding and modulate receptor affinity. J. Biol. Chem. 271, 11090–11098 - PubMed
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

