Nanometer-Scale Chemistry of a Calcite Biomineralization Template: Implications for Skeletal Composition and Nucleation
- PMID: 27794119
- PMCID: PMC5135321
- DOI: 10.1073/pnas.1522864113
Nanometer-Scale Chemistry of a Calcite Biomineralization Template: Implications for Skeletal Composition and Nucleation
Abstract
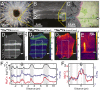
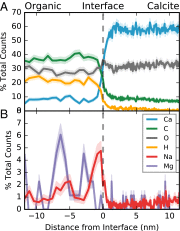
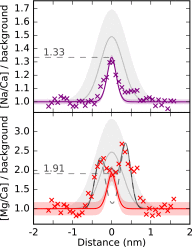
Plankton, corals, and other organisms produce calcium carbonate skeletons that are integral to their survival, form a key component of the global carbon cycle, and record an archive of past oceanographic conditions in their geochemistry. A key aspect of the formation of these biominerals is the interaction between organic templating structures and mineral precipitation processes. Laboratory-based studies have shown that these atomic-scale processes can profoundly influence the architecture and composition of minerals, but their importance in calcifying organisms is poorly understood because it is difficult to measure the chemistry of in vivo biomineral interfaces at spatially relevant scales. Understanding the role of templates in biomineral nucleation, and their importance in skeletal geochemistry requires an integrated, multiscale approach, which can place atom-scale observations of organic-mineral interfaces within a broader structural and geochemical context. Here we map the chemistry of an embedded organic template structure within a carbonate skeleton of the foraminifera Orbulina universa using both atom probe tomography (APT), a 3D chemical imaging technique with Ångström-level spatial resolution, and time-of-flight secondary ionization mass spectrometry (ToF-SIMS), a 2D chemical imaging technique with submicron resolution. We quantitatively link these observations, revealing that the organic template in O. universa is uniquely enriched in both Na and Mg, and contributes to intraskeletal chemical heterogeneity. Our APT analyses reveal the cation composition of the organic surface, offering evidence to suggest that cations other than Ca2+, previously considered passive spectator ions in biomineral templating, may be important in defining the energetics of carbonate nucleation on organic templates.
Keywords: biomineralization; foraminifera; geochemistry; paleoceanography; templating.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Planktic foraminifera form their shells via metastable carbonate phases.Nat Commun. 2017 Nov 2;8(1):1265. doi: 10.1038/s41467-017-00955-0. Nat Commun. 2017. PMID: 29097678 Free PMC article.
-
Foraminifera promote calcification by elevating their intracellular pH.Proc Natl Acad Sci U S A. 2009 Sep 8;106(36):15374-8. doi: 10.1073/pnas.0904306106. Epub 2009 Aug 25. Proc Natl Acad Sci U S A. 2009. PMID: 19706891 Free PMC article.
-
A modern scleractinian coral with a two-component calcite-aragonite skeleton.Proc Natl Acad Sci U S A. 2021 Jan 19;118(3):e2013316117. doi: 10.1073/pnas.2013316117. Epub 2020 Dec 15. Proc Natl Acad Sci U S A. 2021. PMID: 33323482 Free PMC article.
-
Atom probe tomography for biomaterials and biomineralization.Acta Biomater. 2022 Aug;148:44-60. doi: 10.1016/j.actbio.2022.06.010. Epub 2022 Jun 13. Acta Biomater. 2022. PMID: 35709940 Review.
-
Biomineralization: Some complex crystallite-oriented skeletal structures.J Biosci. 2013 Dec;38(5):925-35. doi: 10.1007/s12038-013-9390-z. J Biosci. 2013. PMID: 24296896 Review.
Cited by
-
Nanoscale trace metal imprinting of biocalcification of planktic foraminifers by Toba's super-eruption.Sci Rep. 2020 Jul 3;10(1):10974. doi: 10.1038/s41598-020-67481-w. Sci Rep. 2020. PMID: 32620909 Free PMC article.
-
Biomineralization: Integrating mechanism and evolutionary history.Sci Adv. 2022 Mar 11;8(10):eabl9653. doi: 10.1126/sciadv.abl9653. Epub 2022 Mar 9. Sci Adv. 2022. PMID: 35263127 Free PMC article. Review.
-
Atom probe tomography.Nat Rev Methods Primers. 2021;1(51):10.1038/s43586-021-00047-w. doi: 10.1038/s43586-021-00047-w. Nat Rev Methods Primers. 2021. PMID: 37719173 Free PMC article.
-
Greater functional diversity and redundancy of coral endolithic microbiomes align with lower coral bleaching susceptibility.ISME J. 2022 Oct;16(10):2406-2420. doi: 10.1038/s41396-022-01283-y. Epub 2022 Jul 15. ISME J. 2022. PMID: 35840731 Free PMC article.
-
Form and function of F-actin during biomineralization revealed from live experiments on foraminifera.Proc Natl Acad Sci U S A. 2019 Mar 5;116(10):4111-4116. doi: 10.1073/pnas.1810394116. Epub 2019 Feb 19. Proc Natl Acad Sci U S A. 2019. PMID: 30782789 Free PMC article.
References
-
- Knoll A. Biomineralization and evolutionary history. Rev Mineral Geochem. 2003;54(1):329–356.
-
- Elderfield H. Climate change. Carbonate mysteries. Science. 2002;296(5573):1618–1621. - PubMed
-
- Katz ME, Cramer BS, Franzese A, Hönisch B. Traditional and emerging geochemical proxies in foraminifera. J Foraminiferal Res. 2010;40(2):165–192.
-
- de Yoreo JJ, Wierzbicki A, Dove PM. New insights into mechanisms of biomolecular control on growth of inorganic crystals. CrystEngComm. 2007;9(12):1144–1152.
-
- Gilbert PUPA, Abrecht M, Frazer BH. The organic-mineral interface in biominerals. Rev Mineral Geochem. 2005;59(1):157–185.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

