Infection with the Lyme disease pathogen suppresses innate immunity in mice with diet-induced obesity
- PMID: 27794208
- PMCID: PMC5383418
- DOI: 10.1111/cmi.12689
Infection with the Lyme disease pathogen suppresses innate immunity in mice with diet-induced obesity
Abstract
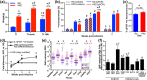
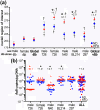
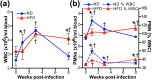
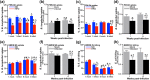
Obesity is a major global public health concern. Immune responses implicated in obesity also control certain infections. We investigated the effects of high-fat diet-induced obesity (DIO) on infection with the Lyme disease bacterium Borrelia burgdorferi in mice. DIO was associated with systemic suppression of neutrophil- and macrophage-based innate immune responses. These included bacterial uptake and cytokine production, and systemic, progressive impairment of bacterial clearance, and increased carditis severity. B. burgdorferi-infected mice fed normal diet also gained weight at the same rate as uninfected mice fed high-fat diet, toll-like receptor 4 deficiency rescued bacterial clearance defects, which greater in female than male mice, and killing of an unrelated bacterium (Escherichia coli) by bone marrow-derived macrophages from obese, B. burgdorferi-infected mice was also affected. Importantly, innate immune suppression increased with infection duration and depended on cooperative and synergistic interactions between DIO and B. burgdorferi infection. Thus, obesity and B. burgdorferi infection cooperatively and progressively suppressed innate immunity in mice.
Keywords: Borrelia burgdorferi; Lyme disease; diseases; host obesity; immunology; infection.
© 2016 The Authors Cellular Microbiology Published by John Wiley & Sons Ltd.
Figures

References
-
- Amar, S. , Zhou, Q. , Shaik‐Dasthagirisaheb, Y. , & Leeman, S. (2007). Diet‐induced obesity in mice causes changes in immune responses and bone loss manifested by bacterial challenge. Proceedings of the National Academy of Sciences of the United States of America, 104(51), 20466–20471. doi:10.1073/pnas.0710335105 - DOI - PMC - PubMed
-
- Barthold, S. W. , Cadavid, D. , & Philipp, M. T. (2010). Animal models of borreliosis In Radolf J. D., & Samuels D. S. (Eds.), Borrelia: Molecular biology, host interaction, and pathogenesis. (pp. 359–411). Norfolk, UK: Caister Academic Press.
-
- Barthold, S. W. , Sidman, C. L. , & Smith, A. L. (1992). Lyme borreliosis in genetically resistant and susceptible mice with severe combined immunodeficiency. The American Journal of Tropical Medicine and Hygiene, 47(5), 605–613. - PubMed
-
- Benhnia, M. R. , Wroblewski, D. , Akhtar, M. N. , Patel, R. A. , Lavezzi, W. , Gangloff, S. C. , … Sellati, T. J. (2005). Signaling through CD14 attenuates the inflammatory response to Borrelia burgdorferi, the agent of Lyme disease. Journal of Immunology, 174(3), 1539–1548. doi:10.4049/jimmunol.174.3.1539 - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

