Choriocapillaris Nonperfusion is Associated With Poor Visual Acuity in Eyes With Reticular Pseudodrusen
- PMID: 27794427
- PMCID: PMC5253325
- DOI: 10.1016/j.ajo.2016.10.005
Choriocapillaris Nonperfusion is Associated With Poor Visual Acuity in Eyes With Reticular Pseudodrusen
Abstract
Purpose: To study choriocapillaris blood flow in age-related macular degeneration (AMD) using optical coherence tomography angiography (OCTA) and study its correlation to visual acuity (VA) in eyes with reticular pseudodrusen (RPD) vs those with drusen without RPD (drusen).
Design: Cross-sectional study.
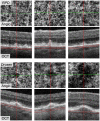
Methods: Patients with either drusen or RPD in early AMD underwent OCTA imaging of the superior, inferior, and/or nasal macula. We quantified "percent choriocapillaris area of nonperfusion" (PCAN) in eyes with RPD vs those with drusen. We assessed the repeatability of PCAN and its correlations with VA.
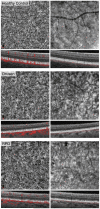
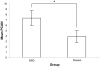
Results: Twenty-nine eyes of 26 patients with RPD and 21 eyes of 16 age-matched AMD patients with drusen were included. Qualitatively, the choriocapillaris in areas with RPD showed focal dark regions without flow signal on OCTA (nonperfusion). The repeatability coefficient of PCAN was 0.49%. Eyes with RPD had significantly greater PCAN compared with eyes with drusen (7.31% and 3.88%, respectively; P < .001). We found a significant correlation between PCAN and VA for the entire dataset (r = 0.394, P = .005). When considering eyes with RPD separately, this correlation was stronger (r = 0.474, P = .009) but lost significance when considering eyes with drusen separately (r = 0.175, P = .45).
Conclusions: Eyes with RPD have significantly larger areas of choriocapillaris nonperfusion compared with eyes with drusen and no RPD. The correlation between PCAN and VA in this RPD population provides a potential mechanistic explanation for vision compromise in RPD compared with other forms of drusen in AMD.
Copyright © 2016 Elsevier Inc. All rights reserved.
Figures



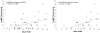

References
-
- Vingerling JR, Dielemans I, Hofman A, et al. The prevalence of age-related maculopathy in the Rotterdam Study. Ophthalmology. 1995;102(2):205–210. - PubMed
-
- Hawkins BS, Bird A, Klein R, West SK. Epidemiology of age-related macular degeneration. Mol Vis. 1999;5(26):b1. - PubMed
-
- Lamoureux EL, Mitchell P, Rees G, et al. Impact of early and late age-related macular degeneration on vision-specific functioning. Br J Ophthalmol. 2010bjo. 2010.185207. - PubMed
-
- Steinberg JS, Fitzke FW, Fimmers R, Fleckenstein M, Holz FG, Schmitz-Valckenberg S. Scotopic and photopic microperimetry in patients with reticular drusen and age-related macular degeneration. JAMA Ophthalmol. 2015;133(6):690–697. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

