PINK1 and Parkin are genetic modifiers for FUS-induced neurodegeneration
- PMID: 27794540
- PMCID: PMC6078632
- DOI: 10.1093/hmg/ddw310
PINK1 and Parkin are genetic modifiers for FUS-induced neurodegeneration
Abstract
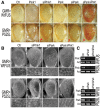
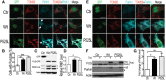
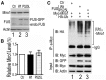
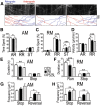
Dysregulation of Fused in Sarcoma (FUS) gene expression is associated with fronto-temporal lobar degeneration (FTLD), and missense mutations in the FUS gene have been identified in patients affected by amyotrophic lateral sclerosis (ALS). However, molecular and cellular defects underlying FUS proteinopathy remain to be elucidated. Here, we examined whether genes important for mitochondrial quality control play a role in FUS proteinopathy. In our genetic screening, Pink1 and Park genes were identified as modifiers of neurodegeneration phenotypes induced by wild type (Wt) or ALS-associated P525L-mutant human FUS. Down-regulating expression of either Pink1 or Parkin genes ameliorated FUS-induced neurodegeneration phenotypes. The protein levels of PINK1 and Parkin were elevated in cells overexpressing FUS. Remarkably, ubiquitinylation of Miro1 protein, a downstream target of the E3 ligase activity of Parkin, was also increased in cells overexpressing FUS protein. In fly motor neurons expressing FUS, both motility and processivity of mitochondrial axonal transport were reduced by expression of either Wt- or P525L-mutant FUS. Finally, down-regulating PINK1 or Parkin partially rescued the locomotive defects and enhanced the survival rate in transgenic flies expressing FUS. Our data indicate that PINK1 and Parkin play an important role in FUS-induced neurodegeneration. This study has uncovered a previously unknown link between FUS proteinopathy and PINK1/Parkin genes, providing new insights into the pathogenesis of FUS proteinopathy.
© The Author 2016. Published by Oxford University Press. All rights reserved. For Permissions, please email: journals.permissions@oup.com.
Figures
References
-
- Kwiatkowski T.J., Jr., Bosco D.A., Leclerc A.L., Tamrazian E., Vanderburg C.R., Russ C., Davis A., Gilchrist J., Kasarskis E.J., Munsat T. et al. (2009) Mutations in the FUS/TLS gene on chromosome 16 cause familial amyotrophic lateral sclerosis. Science, 323, 1205–1208. - PubMed
-
- Lattante S., Rouleau G.A., Kabashi E. (2013) TARDBP and FUS mutations associated with amyotrophic lateral sclerosis: summary and update. Hum. Mutat., 34, 812–826. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

