Informing Antibiotic Treatment Decisions: Evaluating Rapid Molecular Diagnostics To Identify Susceptibility and Resistance to Carbapenems against Acinetobacter spp. in PRIMERS III
- PMID: 27795336
- PMCID: PMC5228224
- DOI: 10.1128/JCM.01524-16
Informing Antibiotic Treatment Decisions: Evaluating Rapid Molecular Diagnostics To Identify Susceptibility and Resistance to Carbapenems against Acinetobacter spp. in PRIMERS III
Erratum in
-
Correction for Evans et al., "Informing Antibiotic Treatment Decisions: Evaluating Rapid Molecular Diagnostics To Identify Susceptibility and Resistance to Carbapenems against Acinetobacter spp. in PRIMERS III".J Clin Microbiol. 2017 Jun;55(6):1970. doi: 10.1128/JCM.00525-17. J Clin Microbiol. 2017. PMID: 28536161 Free PMC article. No abstract available.
Abstract
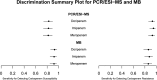
The widespread dissemination of carbapenem-resistant Acinetobacter spp. has created significant therapeutic challenges. At present, rapid molecular diagnostics (RMDs) that can identify this phenotype are not commercially available. Two RMD platforms, PCR combined with electrospray ionization mass spectrometry (PCR/ESI-MS) and molecular beacons (MB), for detecting genes conferring resistance/susceptibility to carbapenems in Acinetobacter spp. were evaluated. An archived collection of 200 clinical Acinetobacter sp. isolates was tested. Predictive values for susceptibility and resistance were estimated as a function of susceptibility prevalence and were based on the absence or presence of beta-lactamase (bla) NDM, VIM, IMP, KPC, and OXA carbapenemase genes (e.g., blaOXA-23, blaOXA-24/40, and blaOXA-58 found in this study) against the reference standard of MIC determinations. According to the interpretation of MICs, 49% (n = 98) of the isolates were carbapenem resistant (as defined by either resistance or intermediate resistance to imipenem). The susceptibility sensitivities (95% confidence interval [CI]) for imipenem were 82% (74%, 89%) and 92% (85%, 97%) for PCR/ESI-MS and MB, respectively. Resistance sensitivities (95% CI) for imipenem were 95% (88%, 98%) and 88% (80%, 94%) for PCR/ESI-MS and MB, respectively. PRIMERS III establishes that RMDs can discriminate between carbapenem resistance and susceptibility in Acinetobacter spp. In the context of a known prevalence of resistance, SPVs and RPVs can inform clinicians regarding the best choice for empiric antimicrobial therapy against this multidrug-resistant pathogen.
Keywords: Acinetobacter; beta-lactams; carbapenemases.
Copyright © 2016 American Society for Microbiology.
Figures


References
-
- Evans SR, Hujer AM, Jiang H, Hujer KM, Hall T, Marzan C, Jacobs MR, Sampath R, Ecker DJ, Manca C, Chavda K, Zhang P, Fernandez H, Chen L, Mediavilla JR, Hill CB, Perez F, Caliendo AM, Fowler VG Jr, Chambers HF, Kreiswirth BN, Bonomo RA. 2016. Rapid molecular diagnostics, antibiotic treatment decisions, and developing approaches to inform empiric therapy: PRIMERS I and II. Clin Infect Dis 62:181–189. doi:10.1093/cid/civ837. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

