Arginase Is Essential for Survival of Leishmania donovani Promastigotes but Not Intracellular Amastigotes
- PMID: 27795357
- PMCID: PMC5203656
- DOI: 10.1128/IAI.00554-16
Arginase Is Essential for Survival of Leishmania donovani Promastigotes but Not Intracellular Amastigotes
Abstract
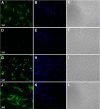
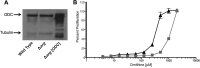
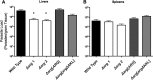
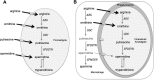
Studies of Leishmania donovani have shown that both ornithine decarboxylase and spermidine synthase, two enzymes of the polyamine biosynthetic pathway, are critical for promastigote proliferation and required for maximum infection in mice. However, the importance of arginase (ARG), the first enzyme of the polyamine pathway in Leishmania, has not been analyzed in L. donovani To test ARG function in intact parasites, we generated Δarg null mutants in L. donovani and evaluated their ability to proliferate in vitro and trigger infections in mice. The Δarg knockout was incapable of growth in the absence of polyamine supplementation, but the auxotrophic phenotype could be bypassed by addition of either millimolar concentrations of ornithine or micromolar concentrations of putrescine or by complementation with either glycosomal or cytosolic versions of ARG. Spermidine supplementation of the medium did not circumvent the polyamine auxotrophy of the Δarg line. Although ARG was found to be essential for ornithine and polyamine synthesis, ornithine decarboxylase appeared to be the rate-limiting enzyme for polyamine production. Mouse infectivity studies revealed that the Δarg lesion reduced parasite burdens in livers by an order of magnitude but had little impact on the numbers of parasites recovered from spleens. Thus, ARG is essential for proliferation of promastigotes but not intracellular amastigotes. Coupled with previous studies, these data support a model in which L. donovani amastigotes readily salvage ornithine and have some access to host spermidine pools, while host putrescine appears to be unavailable for salvage by the parasite.
Keywords: Leishmania; arginase; polyamines.
Copyright © 2016 Boitz et al.
Figures



References
-
- Kedzierski L. 2011. Leishmaniasis. Hum Vaccin 7:1204–1214. - PubMed
-
- Muller R. 2007. Advances in parasitology, vol 65. Academic Press, San Diego, CA.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

