Escherichia coli Probiotic Strain ED1a in Pigs Has a Limited Impact on the Gut Carriage of Extended-Spectrum-β-Lactamase-Producing E. coli
- PMID: 27795372
- PMCID: PMC5192156
- DOI: 10.1128/AAC.01293-16
Escherichia coli Probiotic Strain ED1a in Pigs Has a Limited Impact on the Gut Carriage of Extended-Spectrum-β-Lactamase-Producing E. coli
Abstract
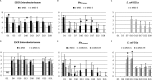
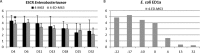
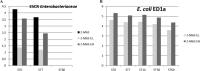
Four trials were conducted to evaluate the impact of Escherichia coli probiotic strain ED1a administration to pigs on the gut carriage or survival in manure of extended-spectrum-β-lactamase-producing E. coli Groups of pigs were orally inoculated with strain E. coli M63 carrying the blaCTX-M-1 gene (n = 84) or used as a control (n = 26). In the first two trials, 24 of 40 E. coli M63-inoculated pigs were given E. coli ED1a orally for 6 days starting 8 days after oral inoculation. In the third trial, 10 E. coli M63-inoculated pigs were given either E. coli ED1a or probiotic E. coli Nissle 1917 for 5 days. In the fourth trial, E. coli ED1a was given to a sow and its 12 piglets, and these 12 piglets plus 12 piglets that had not received E. coli ED1a were then inoculated with E. coli M63. Fecal shedding of cefotaxime-resistant Enterobacteriaceae (CTX-RE) was studied by culture, and blaCTX-M-1 genes were quantified by PCR. The persistence of CTX-RE in manure samples from inoculated pigs or manure samples inoculated in vitro with E. coli M63 with or without probiotics was studied. The results showed that E. coli M63 and ED1a were good gut colonizers. The reduction in the level of fecal excretion of CTX-RE in E. coli ED1a-treated pigs compared to that in nontreated pigs was usually less than 1 log10 CFU and was mainly observed during the probiotic administration period. The results obtained with E. coli Nissle 1917 did not differ significantly from those obtained with E. coli ED1a. CTX-RE survival did not differ significantly in manure samples with or without probiotic treatment. In conclusion, under our experimental conditions, E. coli ED1a and E. coli Nissle 1917 could not durably prevent CTX-RE colonization of the pig gut.
Keywords: antibiotic resistance; cephalosporin; cephalosporin resistance; microbiota; pig; probiotic.
Copyright © 2016 American Society for Microbiology.
Figures


References
-
- ANSES. 2013. Réseau d'épidémiosurveillance de l'antibiorésistance des bactéries pathogènes animales: Bilan 2012. ANSES, Ploufragan, France: http://www.resapath.anses.fr/resapath_uploadfiles/files/Documents/2012%2....
-
- Panel on Biological Hazards. 2011. Scientific opinion on the public health risks of bacterial strains producing extended-spectrum β-lactamases and/or AmpC β-lactamases in food and food-producing animals. EFSA J 9:1. doi: 10.2903/j.efsa.2011.2322. - DOI
-
- Hu YY, Cai JC, Zhou HW, Chi D, Zhang XF, Chen WL, Zhang R, Chen GX. 2013. Molecular typing of CTX-M-producing Escherichia coli isolates from environmental water, swine feces, specimens from healthy humans, and human patients. Appl Environ Microbiol 79:5988–16. doi: 10.1128/AEM.01740-13. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

