Preclinical Characterization and Human Microdose Pharmacokinetics of ITMN-8187, a Nonmacrocyclic Inhibitor of the Hepatitis C Virus NS3 Protease
- PMID: 27795376
- PMCID: PMC5192143
- DOI: 10.1128/AAC.01569-16
Preclinical Characterization and Human Microdose Pharmacokinetics of ITMN-8187, a Nonmacrocyclic Inhibitor of the Hepatitis C Virus NS3 Protease
Abstract
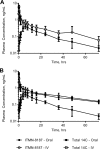
The current paradigm for the treatment of chronic hepatitis C virus (HCV) infection involves combinations of agents that act directly on steps of the HCV life cycle. Here we report the preclinical characteristics of ITMN-8187, a nonmacrocyclic inhibitor of the NS3/4A HCV protease. X-ray crystallographic studies of ITMN-8187 and simeprevir binding to NS3/4A protease demonstrated good agreement between structures. Low nanomolar biochemical potency was maintained against NS3/4A derived from HCV genotypes 1, 2b, 4, 5, and 6. In cell-based potency assays, half-maximal reduction of genotype 1a and 1b HCV replicon RNA was afforded by 11 and 4 nM doses of ITMN-8187, respectively. Combinations of ITMN-8187 with other directly acting antiviral agents in vitro displayed additive antiviral efficacy. A 30-mg/kg of body weight dose of ITMN-8187 administered for 4 days yielded significant viral load reductions through day 5 in a chimeric mouse model of HCV. A 3-mg/kg oral dose administered to rats, dogs, or monkeys yielded concentrations in plasma 16 h after dosing that exceeded the half-maximal effective concentration of ITMN-8187. Human microdose pharmacokinetics showed low intersubject variability and prolonged oral absorption with first-order elimination kinetics compatible with once-daily dosing. These preclinical characteristics compare favorably with those of other NS3/4A inhibitors approved for the treatment of chronic HCV infection.
Keywords: antiviral agents; hepatitis C virus; protease inhibitors.
Copyright © 2016 American Society for Microbiology.
Figures





References
-
- World Health Organization. July 2016. Hepatitis C fact sheet. http://www.who.int/mediacentre/factsheets/fs164/en.
-
- Centers for Disease Control and Prevention. May 2015. Hepatitis C information. http://www.cdc.gov/hepatitis/HCV/.
-
- Centers for Disease Control and Prevention. June 2012. Viral hepatitis statistics and surveillance: number and rate of deaths with hepatitis C listed as a cause of death, by demographic characteristic and year—United States, 2004-2008. http://www.cdc.gov/hepatitis/Statistics/2010Surveillance/Table4.5.htm.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

