Pharmacodynamics of Aerosolized Fosfomycin and Amikacin against Resistant Clinical Isolates of Pseudomonas aeruginosa and Klebsiella pneumoniae in a Hollow-Fiber Infection Model: Experimental Basis for Combination Therapy
- PMID: 27795380
- PMCID: PMC5192115
- DOI: 10.1128/AAC.01763-16
Pharmacodynamics of Aerosolized Fosfomycin and Amikacin against Resistant Clinical Isolates of Pseudomonas aeruginosa and Klebsiella pneumoniae in a Hollow-Fiber Infection Model: Experimental Basis for Combination Therapy
Abstract
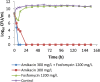
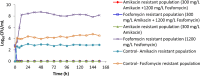
There has been a resurgence of interest in aerosolization of antibiotics for treatment of patients with severe pneumonia caused by multidrug-resistant pathogens. A combination formulation of amikacin-fosfomycin is currently undergoing clinical testing although the exposure-response relationships of these drugs have not been fully characterized. The aim of this study was to describe the individual and combined antibacterial effects of simulated epithelial lining fluid exposures of aerosolized amikacin and fosfomycin against resistant clinical isolates of Pseudomonas aeruginosa (MICs of 16 mg/liter and 64 mg/liter) and Klebsiella pneumoniae (MICs of 2 mg/liter and 64 mg/liter) using a dynamic hollow-fiber infection model over 7 days. Targeted peak concentrations of 300 mg/liter amikacin and/or 1,200 mg/liter fosfomycin as a 12-hourly dosing regimens were used. Quantitative cultures were performed to describe changes in concentrations of the total and resistant bacterial populations. The targeted starting inoculum was 108 CFU/ml for both strains. We observed that neither amikacin nor fosfomycin monotherapy was bactericidal against P. aeruginosa while both were associated with rapid amplification of resistant P. aeruginosa strains (about 108 to 109 CFU/ml within 24 to 48 h). For K. pneumoniae, amikacin but not fosfomycin was bactericidal. When both drugs were combined, a rapid killing was observed for P. aeruginosa and K. pneumoniae (6-log kill within 24 h). Furthermore, the combination of amikacin and fosfomycin effectively suppressed growth of resistant strains of P. aeruginosa and K. pneumoniae In conclusion, the combination of amikacin and fosfomycin was effective at maximizing bacterial killing and suppressing emergence of resistance against these clinical isolates.
Keywords: multidrug resistance; nebulized; pharmacodynamics; pharmacokinetics.
Copyright © 2016 Sime et al.
Figures



References
-
- Jamal JA, Abdul-Aziz MH, Lipman J, Roberts JA. 2013. Defining antibiotic dosing in lung infections. Clin Pulm Med 20:121–128. doi: 10.1097/CPM.0b013e31828fc646. - DOI
-
- Montgomery AB, Vallance S, Abuan T, Tservistas M, Davies A. 2014. A randomized double-blind placebo-controlled dose-escalation phase 1 study of aerosolized amikacin and fosfomycin delivered via the PARI investigational eFlow inline nebulizer system in mechanically ventilated patients. J Aerosol Med Pulm Drug Deliv 27:441–448. doi: 10.1089/jamp.2013.1100. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

