Native Folding of a Recombinant gpE1/gpE2 Heterodimer Vaccine Antigen from a Precursor Protein Fused with Fc IgG
- PMID: 27795422
- PMCID: PMC5165201
- DOI: 10.1128/JVI.01552-16
Native Folding of a Recombinant gpE1/gpE2 Heterodimer Vaccine Antigen from a Precursor Protein Fused with Fc IgG
Abstract
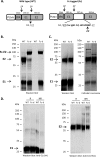
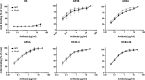
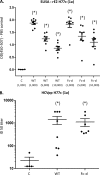
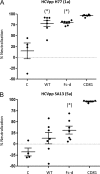
A recombinant strain HCV1 (hepatitis C virus [HCV] genotype 1a) gpE1/gpE2 (E1E2) vaccine candidate was previously shown by our group to protect chimpanzees and generate broad cross-neutralizing antibodies in animals and humans. In addition, recent independent studies have highlighted the importance of conserved neutralizing epitopes in HCV vaccine development that map to antigenic clusters in E2 or the E1E2 heterodimer. E1E2 can be purified using Galanthis nivalis lectin agarose (GNA), but this technique is suboptimal for global production. Our goal was to investigate a high-affinity and scalable method for isolating E1E2. We generated an Fc tag-derived (Fc-d) E1E2 that was selectively captured by protein G Sepharose, with the tag being removed subsequently using PreScission protease. Surprisingly, despite the presence of the large Fc tag, Fc-d E1E2 formed heterodimers similar to those formed by GNA-purified wild-type (WT) E1E2 and exhibited nearly identical binding profiles to HCV monoclonal antibodies that target conserved neutralizing epitopes in E2 (HC33.4, HC84.26, and AR3B) and the E1E2 heterodimer (AR4A and AR5A). Antisera from immunized mice showed that Fc-d E1E2 elicited anti-E2 antibody titers and neutralization of HCV pseudotype viruses similar to those with WT E1E2. Competition enzyme-linked immunosorbent assays (ELISAs) showed that antisera from immunized mice inhibited monoclonal antibody binding to neutralizing epitopes. Antisera from Fc-d E1E2-immunized mice exhibited stronger competition for AR3B and AR5A than the WT, whereas the levels of competition for HC84.26 and AR4A were similar. We anticipate that Fc-d E1E2 will provide a scalable purification and manufacturing process using protein A/G-based chromatography.
Importance: A prophylactic HCV vaccine is still needed to control this global disease despite the availability of direct-acting antivirals. Previously, we demonstrated that a recombinant envelope glycoprotein (E1E2) vaccine (genotype 1a) elicited cross-neutralizing antibodies from human volunteers. A challenge for isolating the E1E2 antigen is the reliance on GNA, which is unsuitable for large scale-up and global vaccine delivery. We have generated a novel Fc domain-tagged E1E2 antigen that forms functional heterodimers similar to those with native E1E2. Affinity purification and removal of the Fc tag from E1E2 resulted in an antigen with a nearly identical profile of cross-neutralizing epitopes. This antigen elicited anti-HCV antibodies that targeted conserved neutralizing epitopes of E1E2. Owing to the high selectivity and cost-effective binding capacity of affinity resins for capture of the Fc-tagged rE1E2, we anticipate that our method will provide a means for large-scale production of this HCV vaccine candidate.
Keywords: envelope glycoproteins; epitopes; hepatitis C virus; neutralizing antibodies; vaccines.
Copyright © 2016 American Society for Microbiology.
Figures

Similar articles
-
Design of a native-like secreted form of the hepatitis C virus E1E2 heterodimer.Proc Natl Acad Sci U S A. 2021 Jan 19;118(3):e2015149118. doi: 10.1073/pnas.2015149118. Proc Natl Acad Sci U S A. 2021. PMID: 33431677 Free PMC article.
-
Recombinant hepatitis C virus envelope glycoprotein vaccine elicits antibodies targeting multiple epitopes on the envelope glycoproteins associated with broad cross-neutralization.J Virol. 2014 Dec;88(24):14278-88. doi: 10.1128/JVI.01911-14. Epub 2014 Oct 1. J Virol. 2014. PMID: 25275133 Free PMC article.
-
Role of the E2 Hypervariable Region (HVR1) in the Immunogenicity of a Recombinant Hepatitis C Virus Vaccine.J Virol. 2018 May 14;92(11):e02141-17. doi: 10.1128/JVI.02141-17. Print 2018 Jun 1. J Virol. 2018. PMID: 29540595 Free PMC article.
-
Hepatitis C Virus E1E2 Structure, Diversity, and Implications for Vaccine Development.Viruses. 2024 May 18;16(5):803. doi: 10.3390/v16050803. Viruses. 2024. PMID: 38793684 Free PMC article. Review.
-
Structural and Biophysical Characterization of the HCV E1E2 Heterodimer for Vaccine Development.Viruses. 2021 May 29;13(6):1027. doi: 10.3390/v13061027. Viruses. 2021. PMID: 34072451 Free PMC article. Review.
Cited by
-
From Structural Studies to HCV Vaccine Design.Viruses. 2021 May 4;13(5):833. doi: 10.3390/v13050833. Viruses. 2021. PMID: 34064532 Free PMC article. Review.
-
Hepatitis C Virus Vaccine Research: Time to Put Up or Shut Up.Viruses. 2021 Aug 12;13(8):1596. doi: 10.3390/v13081596. Viruses. 2021. PMID: 34452460 Free PMC article. Review.
-
Design of a native-like secreted form of the hepatitis C virus E1E2 heterodimer.Proc Natl Acad Sci U S A. 2021 Jan 19;118(3):e2015149118. doi: 10.1073/pnas.2015149118. Proc Natl Acad Sci U S A. 2021. PMID: 33431677 Free PMC article.
-
Effect of Different Adjuvants on the Longevity and Strength of Humoral and Cellular Immune Responses to the HCV Envelope Glycoproteins.Vaccines (Basel). 2019 Dec 3;7(4):204. doi: 10.3390/vaccines7040204. Vaccines (Basel). 2019. PMID: 31816920 Free PMC article.
-
Chlorcyclizine Inhibits Viral Fusion of Hepatitis C Virus Entry by Directly Targeting HCV Envelope Glycoprotein 1.Cell Chem Biol. 2020 Jul 16;27(7):780-792.e5. doi: 10.1016/j.chembiol.2020.04.006. Epub 2020 May 7. Cell Chem Biol. 2020. PMID: 32386595 Free PMC article.
References
-
- Midgard H, Bjoro B, Maeland A, Konopski Z, Kileng H, Damas JK, Paulsen J, Heggelund L, Sandvei PK, Ringstad JO, Karlsen LN, Stene-Johansen K, Pettersson JH, Dorenberg DH, Dalgard O. 2016. Hepatitis C reinfection after sustained virological response. J Hepatol 64:1020–1026. doi:10.1016/j.jhep.2016.01.001. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

