Host Cellular Protein TRAPPC6AΔ Interacts with Influenza A Virus M2 Protein and Regulates Viral Propagation by Modulating M2 Trafficking
- PMID: 27795429
- PMCID: PMC5165196
- DOI: 10.1128/JVI.01757-16
Host Cellular Protein TRAPPC6AΔ Interacts with Influenza A Virus M2 Protein and Regulates Viral Propagation by Modulating M2 Trafficking
Abstract
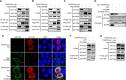
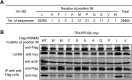
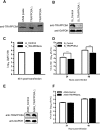
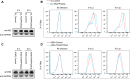
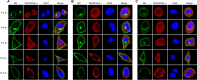
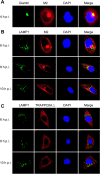
Influenza A virus (IAV) matrix protein 2 (M2) plays multiple roles in the early and late phases of viral infection. Once synthesized, M2 is translocated to the endoplasmic reticulum (ER), travels to the Golgi apparatus, and is sorted at the trans-Golgi network (TGN) for transport to the apical plasma membrane, where it functions in virus budding. We hypothesized that M2 trafficking along with its secretory pathway must be finely regulated, and host factors could be involved in this process. However, no studies examining the role of host factors in M2 posttranslational transport have been reported. Here, we used a yeast two-hybrid (Y2H) system to screen for host proteins that interact with the M2 protein and identified transport protein particle complex 6A (TRAPPC6A) as a potential binding partner. We found that both TRAPPC6A and its N-terminal internal-deletion isoform, TRAPPC6A delta (TRAPPC6AΔ), interact with M2. Truncation and mutation analyses showed that the highly conserved leucine residue at position 96 of M2 is critical for mediating this interaction. The role of TRAPPC6AΔ in the viral life cycle was investigated by the knockdown of endogenous TRAPPC6AΔ with small interfering RNA (siRNA) and by generating a recombinant virus that was unable to interact with TRAPPC6A/TRAPPC6AΔ. The results indicated that TRAPPC6AΔ, through its interaction with M2, slows M2 trafficking to the apical plasma membrane, favors viral replication in vitro, and positively modulates virus virulence in mice.
Importance: The influenza A virus M2 protein regulates the trafficking of not only other proteins but also itself along the secretory pathway. However, the host factors involved in the regulation of the posttranslational transport of M2 are largely unknown. In this study, we identified TRAPPC6A and its N-terminal internal-deletion isoform, TRAPPC6AΔ, as interacting partners of M2. We found that the leucine (L) residue at position 96 of M2 is critical for mediating this interaction, which leads us to propose that the high level of conservation of 96L is a consequence of M2 adaptation to its interacting host factor TRAPPC6A/TRAPPC6AΔ. Importantly, we discovered that TRAPPC6AΔ can positively regulate viral replication in vitro by modulating M2 trafficking to the plasma membrane.
Keywords: M2; TRAPPC6AΔ; influenza A virus; transport.
Copyright © 2016 Zhu et al.
Figures




Similar articles
-
Influenza A Virus M2 Protein Apical Targeting Is Required for Efficient Virus Replication.J Virol. 2018 Oct 29;92(22):e01425-18. doi: 10.1128/JVI.01425-18. Print 2018 Nov 15. J Virol. 2018. PMID: 30158290 Free PMC article.
-
Influenza virus matrix protein M1 interacts with SLD5 to block host cell cycle.Cell Microbiol. 2019 Aug;21(8):e13038. doi: 10.1111/cmi.13038. Epub 2019 May 16. Cell Microbiol. 2019. PMID: 31050118
-
The role of nuclear NS1 protein in highly pathogenic H5N1 influenza viruses.Microbes Infect. 2017 Dec;19(12):587-596. doi: 10.1016/j.micinf.2017.08.011. Epub 2017 Sep 10. Microbes Infect. 2017. PMID: 28903072
-
Host microRNAs and exosomes that modulate influenza virus infection.Virus Res. 2020 Apr 2;279:197885. doi: 10.1016/j.virusres.2020.197885. Epub 2020 Jan 22. Virus Res. 2020. PMID: 31981772 Review.
-
[Functional analysis of Host proteins involved in RNA virus replication].Uirusu. 2018;68(1):71-78. doi: 10.2222/jsv.68.71. Uirusu. 2018. PMID: 31105137 Review. Japanese.
Cited by
-
Influenza A Virus-Host Protein Interactions Control Viral Pathogenesis.Int J Mol Sci. 2017 Aug 1;18(8):1673. doi: 10.3390/ijms18081673. Int J Mol Sci. 2017. PMID: 28763020 Free PMC article. Review.
-
Influenza A Virus M2 Protein: Roles from Ingress to Egress.Int J Mol Sci. 2017 Dec 7;18(12):2649. doi: 10.3390/ijms18122649. Int J Mol Sci. 2017. PMID: 29215568 Free PMC article. Review.
-
Caspase cleavage of influenza A virus M2 disrupts M2-LC3 interaction and regulates virion production.EMBO Rep. 2025 Apr;26(7):1768-1791. doi: 10.1038/s44319-025-00388-7. Epub 2025 Mar 3. EMBO Rep. 2025. PMID: 40033051 Free PMC article.
-
Influenza A virus use of BinCARD1 to facilitate the binding of viral NP to importin α7 is counteracted by TBK1-p62 axis-mediated autophagy.Cell Mol Immunol. 2022 Oct;19(10):1168-1184. doi: 10.1038/s41423-022-00906-w. Epub 2022 Sep 2. Cell Mol Immunol. 2022. PMID: 36056146 Free PMC article.
-
A Glu-Glu-Tyr Sequence in the Cytoplasmic Tail of the M2 Protein Renders Influenza A Virus Susceptible to Restriction of the Hemagglutinin-M2 Association in Primary Human Macrophages.J Virol. 2022 Sep 28;96(18):e0071622. doi: 10.1128/jvi.00716-22. Epub 2022 Sep 13. J Virol. 2022. PMID: 36098511 Free PMC article.
References
-
- Jagger BW, Wise HM, Kash JC, Walters KA, Wills NM, Xiao YL, Dunfee RL, Schwartzman LM, Ozinsky A, Bell GL, Dalton RM, Lo A, Efstathiou S, Atkins JF, Firth AE, Taubenberger JK, Digard P. 2012. An overlapping protein-coding region in influenza A virus segment 3 modulates the host response. Science 337:199–204. doi:10.1126/science.1222213. - DOI - PMC - PubMed
-
- Wise HM, Hutchinson EC, Jagger BW, Stuart AD, Kang ZH, Robb N, Schwartzman LM, Kash JC, Fodor E, Firth AE, Gog JR, Taubenberger JK, Digard P. 2012. Identification of a novel splice variant form of the influenza A virus M2 ion channel with an antigenically distinct ectodomain. PLoS Pathog 8:e1002998. doi:10.1371/journal.ppat.1002998. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

