Diversity of fungal feruloyl esterases: updated phylogenetic classification, properties, and industrial applications
- PMID: 27795736
- PMCID: PMC5084320
- DOI: 10.1186/s13068-016-0651-6
Diversity of fungal feruloyl esterases: updated phylogenetic classification, properties, and industrial applications
Abstract
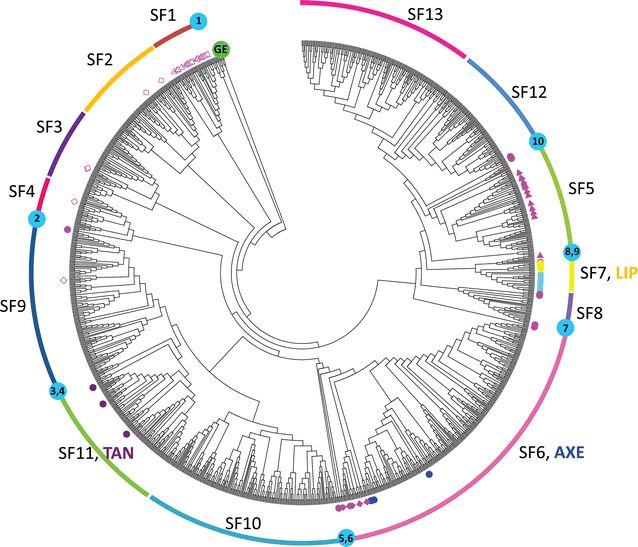
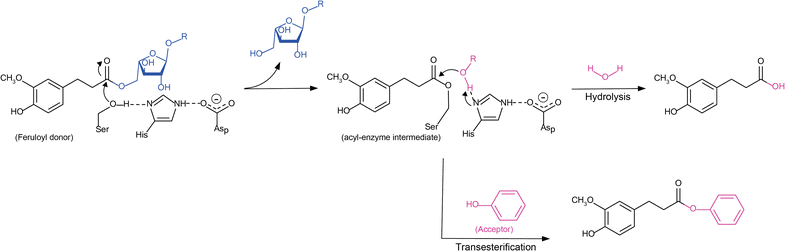
Feruloyl esterases (FAEs) represent a diverse group of carboxyl esterases that specifically catalyze the hydrolysis of ester bonds between ferulic (hydroxycinnamic) acid and plant cell wall polysaccharides. Therefore, FAEs act as accessory enzymes to assist xylanolytic and pectinolytic enzymes in gaining access to their site of action during biomass conversion. Their ability to release ferulic acid and other hydroxycinnamic acids from plant biomass makes FAEs potential biocatalysts in a wide variety of applications such as in biofuel, food and feed, pulp and paper, cosmetics, and pharmaceutical industries. This review provides an updated overview of the knowledge on fungal FAEs, in particular describing their role in plant biomass degradation, diversity of their biochemical properties and substrate specificities, their regulation and conditions needed for their induction. Furthermore, the discovery of new FAEs using genome mining and phylogenetic analysis of current publicly accessible fungal genomes will also be presented. This has led to a new subfamily classification of fungal FAEs that takes into account both phylogeny and substrate specificity.
Keywords: Applications; Biotechnology; Cinnamic acid; Ferulic acid; Feruloyl esterase; Hydroxycinnamic acid; P-coumaric acid; Phylogenetic analysis; Plant cell wall.
Figures


Similar articles
-
Functional Validation of Two Fungal Subfamilies in Carbohydrate Esterase Family 1 by Biochemical Characterization of Esterases From Uncharacterized Branches.Front Bioeng Biotechnol. 2020 Jun 26;8:694. doi: 10.3389/fbioe.2020.00694. eCollection 2020. Front Bioeng Biotechnol. 2020. PMID: 32671051 Free PMC article.
-
Fungal feruloyl esterases: Functional validation of genome mining based enzyme discovery including uncharacterized subfamilies.N Biotechnol. 2018 Mar 25;41:9-14. doi: 10.1016/j.nbt.2017.11.004. Epub 2017 Nov 23. N Biotechnol. 2018. PMID: 29174720
-
Characterization of a feruloyl esterase from Aspergillus terreus facilitates the division of fungal enzymes from Carbohydrate Esterase family 1 of the carbohydrate-active enzymes (CAZy) database.Microb Biotechnol. 2018 Sep;11(5):869-880. doi: 10.1111/1751-7915.13273. Epub 2018 Apr 26. Microb Biotechnol. 2018. PMID: 29697197 Free PMC article.
-
Biotechnological applications and potential of fungal feruloyl esterases based on prevalence, classification and biochemical diversity.Biotechnol Lett. 2008 Mar;30(3):387-96. doi: 10.1007/s10529-007-9564-6. Epub 2007 Nov 1. Biotechnol Lett. 2008. PMID: 17973091 Review.
-
Occurrence, properties, and applications of feruloyl esterases.Appl Microbiol Biotechnol. 2009 Oct;84(5):803-10. doi: 10.1007/s00253-009-2148-8. Epub 2009 Jul 31. Appl Microbiol Biotechnol. 2009. PMID: 19644688 Review.
Cited by
-
Properties of Two Novel Esterases Identified from Culture Supernatant of Penicillium purpurogenum Grown on Sugar Beet Pulp.Insights Enzym Res. 2016;1(1):4. Epub 2016 Dec 12. Insights Enzym Res. 2016. PMID: 28828411 Free PMC article.
-
Fantastic Ferulic Acid Esterases and Their Functions.Int J Mol Sci. 2025 Aug 2;26(15):7474. doi: 10.3390/ijms26157474. Int J Mol Sci. 2025. PMID: 40806602 Free PMC article. Review.
-
Functional Validation of Two Fungal Subfamilies in Carbohydrate Esterase Family 1 by Biochemical Characterization of Esterases From Uncharacterized Branches.Front Bioeng Biotechnol. 2020 Jun 26;8:694. doi: 10.3389/fbioe.2020.00694. eCollection 2020. Front Bioeng Biotechnol. 2020. PMID: 32671051 Free PMC article.
-
Optimized Enzymatic Synthesis of Feruloyl Derivatives Catalyzed by Three Novel Feruloyl Esterases from Talaromyces wortmannii in Detergentless Microemulsions.Comput Struct Biotechnol J. 2018 Oct 5;16:361-369. doi: 10.1016/j.csbj.2018.09.005. eCollection 2018. Comput Struct Biotechnol J. 2018. PMID: 30364734 Free PMC article.
-
Feruloyl Esterases for Biorefineries: Subfamily Classified Specificity for Natural Substrates.Front Bioeng Biotechnol. 2020 Apr 23;8:332. doi: 10.3389/fbioe.2020.00332. eCollection 2020. Front Bioeng Biotechnol. 2020. PMID: 32391342 Free PMC article.
References
-
- Mortimer JC, Miles GP, Brown DM, Zhang Z, Segura MP, Weimar T, Yu X, Seffen KA, Stephens E, Turner SR, Dupree P. Absence of branches from xylan in Arabidopsis gux mutants reveals potential for simplification of lignocellulosic biomass. Proc Natl Acad Sci USA. 2010;107:17409–17414. doi: 10.1073/pnas.1005456107. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

