Biosynthesis of poly(3-hydroxybutyrateco-3-hydroxy-4-methylvalerate) by Strain Azotobacter chroococcum 7B
- PMID: 27795846
- PMCID: PMC5081702
Biosynthesis of poly(3-hydroxybutyrateco-3-hydroxy-4-methylvalerate) by Strain Azotobacter chroococcum 7B
Abstract
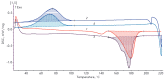
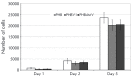
Production of novel polyhydroxyalkanoates (PHAs), biodegradable polymers for biomedical applications, and biomaterials based on them is a promising trend in modern bioengineering. We studied the ability of an effective strain-producer Azotobacter chroococcum 7B to synthesize not only poly(3-hydroxybutyrate) homopolymer (PHB) and its main copolymer poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV), but also a novel copolymer, poly(3-hydroxybutyrate-co-3-hydroxy-4-methylvalerate) (PHB4MV). For the biosynthesis of PHB copolymers, we used carboxylic acids as additional carbon sources and monomer precursors in the chain of synthesized copolymers. The main parameters of these polymers' biosynthesis were determined: strain-producer biomass yield, polymer yield, molecular weight and monomer composition of the synthesized polymers, as well as the morphology of A. chroococcum 7B bacterial cells. The physico-chemical properties of the polymers were studied using nuclear magnetic resonance spectroscopy (NMR), differential scanning calorimetry (DSC), contact angle test, and other methods. In vitro biocompatibility of the obtained polymers was investigated using stromal cells isolated from the bone marrow of rats with the XTT cell viability test. The synthesis of the novel copolymer PHB4MV and its chemical composition were demonstrated by NMR spectroscopy: the addition of 4-methylvaleric acid to the culture medium resulted in incorporation of 3-hydroxy-4-methylvalerate (3H4MV) monomers into the PHB polymer chain (0.6 mol%). Despite the low molar content of 3H4MV in the obtained copolymer, its physico-chemical properties were significantly different from those of the PHB homopolymer: it has lower crystallinity and a higher contact angle, i.e. the physico-chemical properties of the PHB4MV copolymer containing only 0.6 mol% of 3H4MV corresponded to a PHBV copolymer with a molar content ranging from 2.5% to 7.8%. In vitro biocompatibility of the obtained PHB4MV copolymer, measured in the XTT test, was not statistically different from the cell growth of PHB and PHBV polymers, which make its use possible in biomedical research and development.
Keywords: Azotobacter chroococcum 7B; biocompatibility; biosynthesis; bone marrow stromal cells; crystallinity; poly(3-hydroxybutyrate); poly(3-hydroxybutyrate-co-3-hydroxy-4-methylvalerate).
Figures



Similar articles
-
Cell attachment on poly(3-hydroxybutyrate)-poly(ethylene glycol) copolymer produced by Azotobacter chroococcum 7B.BMC Biochem. 2013 May 21;14:12. doi: 10.1186/1471-2091-14-12. BMC Biochem. 2013. PMID: 23692611 Free PMC article.
-
Biosynthesis of poly(3-hydroxybutyrate) copolymers by Azotobacter chroococcum 7B: A precursor feeding strategy.Prep Biochem Biotechnol. 2017 Feb 7;47(2):173-184. doi: 10.1080/10826068.2016.1188317. Epub 2016 May 23. Prep Biochem Biotechnol. 2017. PMID: 27215309
-
The terpolymer produced by Azotobacter chroococcum 7B: effect of surface properties on cell attachment.PLoS One. 2013;8(2):e57200. doi: 10.1371/journal.pone.0057200. Epub 2013 Feb 26. PLoS One. 2013. PMID: 23468935 Free PMC article.
-
Enhancing the Potential of PHAs in Tissue Engineering Applications: A Review of Chemical Modification Methods.Materials (Basel). 2024 Nov 27;17(23):5829. doi: 10.3390/ma17235829. Materials (Basel). 2024. PMID: 39685265 Free PMC article. Review.
-
Development and Advantages of Biodegradable PHA Polymers Based on Electrospun PHBV Fibers for Tissue Engineering and Other Biomedical Applications.ACS Biomater Sci Eng. 2021 Dec 13;7(12):5339-5362. doi: 10.1021/acsbiomaterials.1c00757. Epub 2021 Oct 14. ACS Biomater Sci Eng. 2021. PMID: 34649426 Free PMC article. Review.
Cited by
-
Extended batch cultures for poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV) production by Azotobacter vinelandii OP growing at different aeration rates.3 Biotech. 2022 Nov;12(11):304. doi: 10.1007/s13205-022-03380-3. Epub 2022 Sep 30. 3 Biotech. 2022. PMID: 36276477 Free PMC article.
-
Competitive Biosynthesis of Bacterial Alginate Using Azotobacter vinelandii 12 for Tissue Engineering Applications.Polymers (Basel). 2021 Dec 30;14(1):131. doi: 10.3390/polym14010131. Polymers (Basel). 2021. PMID: 35012152 Free PMC article.
-
Changes in the Gut Microbiota Composition during Implantation of Composite Scaffolds Based on Poly(3-hydroxybutyrate) and Alginate on the Large-Intestine Wall.Polymers (Basel). 2023 Sep 4;15(17):3649. doi: 10.3390/polym15173649. Polymers (Basel). 2023. PMID: 37688275 Free PMC article.
References
-
- Birmingham Jenkins M. Biomedical polymers Ed. Jenkins M. Birmingham, UK: University of Birmingham, 2007. 203 p. 2007. Biomedical polymers.
-
- Shtilman M.I. Polymeric biomaterials. Part 1. Polymer implants. VSP: Leiden, Netherlands, 2003. 294 p. 2003. Polymer implants.
-
- Moisenovich M.M., Pustovalova O., Shackelford J., Vasiljeva T.V., Druzhinina T.V., Kamenchuk Y.A., Guzeev V.V., Sokolova O.S., Bogush V.G., Debabov V.G.. Biomaterials. 2012;33(15):3887–3898. - PubMed
-
- Satyam A., Kumar P., Fan X., Gorelov A., Rochev Y., Joshi L., Peinado H., Lyden D., Thomas B., Rodriguez B.. Advanced Materials. 2014;26(19):3024–3034. - PubMed
-
- Miroiu F.M., Stefan N., Visan A.I., Nita C., Luculescu C.R., Rasoga O., Socol M., Zgura I., Cristescu R., Craciun D., Appl. Surface Sci. 2015;355:1123–1131.
LinkOut - more resources
Full Text Sources
