ATP Induces Disruption of Tight Junction Proteins via IL-1 Beta-Dependent MMP-9 Activation of Human Blood-Brain Barrier In Vitro
- PMID: 27795859
- PMCID: PMC5067334
- DOI: 10.1155/2016/8928530
ATP Induces Disruption of Tight Junction Proteins via IL-1 Beta-Dependent MMP-9 Activation of Human Blood-Brain Barrier In Vitro
Abstract
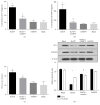
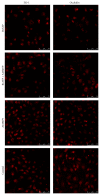
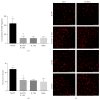
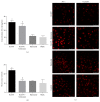
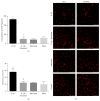
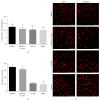
Disruption of blood-brain barrier (BBB) follows brain trauma or central nervous system (CNS) stress. However, the mechanisms leading to this process or the underlying neural plasticity are not clearly known. We hypothesized that ATP/P2X7R signaling regulates the integrity of BBB. Activation of P2X7 receptor (P2X7R) by ATP induces the release of interleukin-1β (IL-1β), which in turn enhances the activity of matrix metalloproteinase-9 (MMP-9). Degradation of tight junction proteins (TJPs) such as ZO-1 and occludin occurs, which finally contributes to disruption of BBB. A contact coculture system using human astrocytes and hCMEC/D3, an immortalized human brain endothelial cell line, was used to mimic BBB in vitro. Permeability was used to evaluate changes in the integrity of TJPs. ELISA, Western blot, and immunofluorescent staining procedures were used. Our data demonstrated that exposure to the photoreactive ATP analog, 3'-O-(4-benzoyl)benzoyl adenosine 5'-triphosphate (BzATP), induced a significant decrease in ZO-1 and occludin expression. Meanwhile, the decrease of ZO-1 and occludin was significantly attenuated by P2X7R inhibitors, as well as IL-1R and MMP antagonists. Further, the induction of IL-1β and MMP-9 was closely linked to ATP/P2X7R-associated BBB leakage. In conclusion, our study explored the mechanism of ATP/P2X7R signaling in the disruption of BBB following brain trauma/stress injury, especially focusing on the relationship with IL-1β and MMP-9.
Figures
Similar articles
-
Melatonin protects blood-brain barrier integrity and permeability by inhibiting matrix metalloproteinase-9 via the NOTCH3/NF-κB pathway.Aging (Albany NY). 2019 Dec 7;11(23):11391-11415. doi: 10.18632/aging.102537. Epub 2019 Dec 7. Aging (Albany NY). 2019. PMID: 31811815 Free PMC article.
-
P2X7 Receptor Suppression Preserves Blood-Brain Barrier through Inhibiting RhoA Activation after Experimental Intracerebral Hemorrhage in Rats.Sci Rep. 2016 Mar 16;6:23286. doi: 10.1038/srep23286. Sci Rep. 2016. PMID: 26980524 Free PMC article.
-
3,4-Methylenedioxymethamphetamine (MDMA, ecstasy) disrupts blood-brain barrier integrity through a mechanism involving P2X7 receptors.Int J Neuropsychopharmacol. 2014 Aug;17(8):1243-55. doi: 10.1017/S1461145714000145. Epub 2014 Mar 14. Int J Neuropsychopharmacol. 2014. PMID: 24626059
-
Recent Advances in the Development of Antidepressants Targeting the Purinergic P2X7 Receptor.Curr Med Chem. 2023;30(2):164-177. doi: 10.2174/0929867329666220629141418. Curr Med Chem. 2023. PMID: 35770396 Review.
-
Modulation of the ATP-lnduced release and processing of IL-1beta in microglial cells.Crit Rev Immunol. 2009;29(4):335-45. doi: 10.1615/critrevimmunol.v29.i4.40. Crit Rev Immunol. 2009. PMID: 19673687 Review.
Cited by
-
Matrix Metalloproteinase-9 as an Important Contributor to the Pathophysiology of Depression.Front Neurol. 2022 Mar 18;13:861843. doi: 10.3389/fneur.2022.861843. eCollection 2022. Front Neurol. 2022. PMID: 35370878 Free PMC article. Review.
-
Pharmacological interventions targeting the microcirculation following traumatic spinal cord injury.Neural Regen Res. 2024 Jan;19(1):35-42. doi: 10.4103/1673-5374.375304. Neural Regen Res. 2024. PMID: 37488841 Free PMC article. Review.
-
Proteomic analysis discovers potential biomarkers of early traumatic axonal injury in the brainstem.Int J Legal Med. 2024 Jan;138(1):207-227. doi: 10.1007/s00414-023-03039-5. Epub 2023 Jun 20. Int J Legal Med. 2024. PMID: 37338605
-
Connexin 43: An Interface Connecting Neuroinflammation to Depression.Molecules. 2023 Feb 15;28(4):1820. doi: 10.3390/molecules28041820. Molecules. 2023. PMID: 36838809 Free PMC article. Review.
-
Discerning the Role of Blood Brain Barrier Dysfunction in Alzheimer's Disease.Aging Dis. 2022 Oct 1;13(5):1391-1404. doi: 10.14336/AD.2022.0130-1. eCollection 2022 Oct 1. Aging Dis. 2022. PMID: 36186141 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

