Interleukin 10 Restores Gastric Emptying, Electrical Activity, and Interstitial Cells of Cajal Networks in Diabetic Mice
- PMID: 27795979
- PMCID: PMC5042607
- DOI: 10.1016/j.jcmgh.2016.04.006
Interleukin 10 Restores Gastric Emptying, Electrical Activity, and Interstitial Cells of Cajal Networks in Diabetic Mice
Abstract
Background & aims: Gastroparesis is a complication of diabetes characterized by delayed emptying of stomach contents and accompanied by early satiety, nausea, vomiting, and pain. No safe and reliable treatments are available. Interleukin 10 (IL10) activates the M2 cytoprotective phenotype of macrophages and induces expression of heme oxygenase 1 (HO1) protein. We investigated whether IL10 administration could improve gastric emptying and reverse the associated cellular and electrical abnormalities in diabetic mice.
Methods: Nonobese diabetic mice with delayed gastric emptying were given either IL10 (0.1-1 μg, twice/day) or vehicle (controls). Stomach tissues were isolated, and sharp microelectrode recordings were made of the electrical activity in the gastric muscle layers. Changes to interstitial cells of Cajal (ICC), reduced nicotinamide adenine dinucleotide phosphate diaphorase, and levels and distribution of HO1 protein were determined by histochemical and imaging analyses of the same tissues.
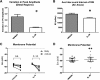
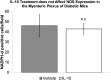
Results: Gastric emptying remained delayed in vehicle-treated diabetic mice but returned to normal in mice given IL10 (n = 10 mice; P < .05). In mice given IL10, normalization of gastric emptying was associated with a membrane potential difference between the proximal and distal stomach, and lower irregularity and higher frequency of slow-wave activity, particularly in the distal stomach. Levels of HO1 protein were higher in stomach tissues from mice given IL10, and ICC networks were more organized, better connected, and more evenly distributed compared with controls.
Conclusions: IL10 increases gastric emptying in diabetic mice and has therapeutic potential for patients with diabetic gastroparesis. This response is associated with up-regulation of HO1 and repair of connectivity of ICC networks.
Keywords: Alternatively Activated Macrophages; CO, carbon monoxide; Electrical Slow Wave; HO1, heme oxygenase 1; Heme Oxygenase 1; ICC, interstitial cells of Cajal; IL10, interleukin 10; MDA, malondialdehyde; NADPH, reduced nicotinamide adenine dinucleotide phosphate; NOD, nonobese diabetic; NOS, nitric oxide synthase; PBS, phosphate-buffered saline.
Figures






Similar articles
-
CD206-positive M2 macrophages that express heme oxygenase-1 protect against diabetic gastroparesis in mice.Gastroenterology. 2010 Jun;138(7):2399-409, 2409.e1. doi: 10.1053/j.gastro.2010.02.014. Epub 2010 Feb 20. Gastroenterology. 2010. PMID: 20178793 Free PMC article.
-
Change in Populations of Macrophages Promotes Development of Delayed Gastric Emptying in Mice.Gastroenterology. 2018 Jun;154(8):2122-2136.e12. doi: 10.1053/j.gastro.2018.02.027. Epub 2018 Mar 6. Gastroenterology. 2018. PMID: 29501441 Free PMC article.
-
Carbon monoxide reverses diabetic gastroparesis in NOD mice.Am J Physiol Gastrointest Liver Physiol. 2010 Jun;298(6):G1013-9. doi: 10.1152/ajpgi.00069.2010. Epub 2010 Apr 8. Am J Physiol Gastrointest Liver Physiol. 2010. PMID: 20378827 Free PMC article.
-
Macrophages in diabetic gastroparesis--the missing link?Neurogastroenterol Motil. 2015 Jan;27(1):7-18. doi: 10.1111/nmo.12418. Epub 2014 Aug 28. Neurogastroenterol Motil. 2015. PMID: 25168158 Free PMC article. Review.
-
Investigational drug therapies for the treatment of gastroparesis.Expert Opin Investig Drugs. 2017 Mar;26(3):331-342. doi: 10.1080/13543784.2017.1288214. Epub 2017 Feb 7. Expert Opin Investig Drugs. 2017. PMID: 28127997 Review.
Cited by
-
Neuroimmune Crossroads: The Interplay of the Enteric Nervous System and Intestinal Macrophages in Gut Homeostasis and Disease.Biomolecules. 2024 Sep 2;14(9):1103. doi: 10.3390/biom14091103. Biomolecules. 2024. PMID: 39334870 Free PMC article. Review.
-
Hyperglycemia Increases Interstitial Cells of Cajal via MAPK1 and MAPK3 Signaling to ETV1 and KIT, Leading to Rapid Gastric Emptying.Gastroenterology. 2017 Aug;153(2):521-535.e20. doi: 10.1053/j.gastro.2017.04.020. Epub 2017 Apr 21. Gastroenterology. 2017. PMID: 28438610 Free PMC article.
-
Adeno-associated virus-9 reverses delayed gastric emptying of solids in diabetic mice.Neurogastroenterol Motil. 2023 Nov;35(11):e14669. doi: 10.1111/nmo.14669. Epub 2023 Sep 13. Neurogastroenterol Motil. 2023. PMID: 37702100 Free PMC article.
-
Gastric Biopsies in Gastroparesis: Insights into Gastric Neuromuscular Disorders to Aid Treatment.Gastroenterol Clin North Am. 2020 Sep;49(3):557-570. doi: 10.1016/j.gtc.2020.04.009. Epub 2020 Jun 14. Gastroenterol Clin North Am. 2020. PMID: 32718570 Free PMC article. Review.
-
High temporal resolution gastric emptying breath tests in mice.Neurogastroenterol Motil. 2018 Mar 25:e13333. doi: 10.1111/nmo.13333. Online ahead of print. Neurogastroenterol Motil. 2018. PMID: 29575442 Free PMC article.
References
-
- Farrell F.J., Keeffe E.B. Diabetic gastroparesis. Dig Dis. 1995;13:291–300. - PubMed
-
- Vittal H., Farrugia G., Gomez G. Mechanisms of disease: the pathological basis of gastroparesis–a review of experimental and clinical studies. Nat Clin Pract Gastroenterol Hepatol. 2007;4:336–346. - PubMed
-
- Camilleri M. Clinical practice. Diabetic gastroparesis. N Engl J Med. 2007;356:820–829. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

