Long Cut Straw Provides Stable the Rates of Survival, Pregnancy and Live Birth for Vitrification of Human Blasotcysts
- PMID: 27796003
- PMCID: PMC5078147
- DOI: 10.12717/DR.2016.20.3.219
Long Cut Straw Provides Stable the Rates of Survival, Pregnancy and Live Birth for Vitrification of Human Blasotcysts
Abstract
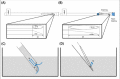
Most of the commercial devices for vitrification are directly immersed into the warming solution (WS) for increasing of warming rate. However, the previous modified cut standard straw (MCS) which has reported is difficult to immerse into the WS. The aim of this study was to investigate whether the long cut straw (LCS) could be useful as a stable tool for vitrified-warmed human blastocysts. A total of 138 vitrified-warmed cycles were performed between November 2013 and November 2014 (exclusion criteria: women ≥38 years old, poor responder, surgical retrieval sperm, and severe male factor). The artificial shrinkage was conducted using 29-gauge needles. Ethylene glycol and dimethyl sulfoxide (7.5% and 15% (v/v)) were used as cryoprotectants. Freezing and warming were conducted using the LCS tool. The cap of LCS was removed using the forceps in the liquid nitrogen (LN2) and then directly immersed into the first WS for 1 min at 37℃ (1 M sucrose). Only re-expanded blastocysts were transferred after it was cultured in sequential media for 18-20 h. A total of 294 blastocysts were warmed, and all were recovered (100%). Two hundred eighty-five embryos were survived (96.9%). The vitrifiedwarmed blastocysts of all patients were transferred without any cancellation. We were able to achieve a reasonable implantation (24.2%), following by clinical pregnancy (36.2%), which then continued to ongoing pregnancy (36.2%), and live birth (31.2%). Using LCS is achieved the acceptable rates of survival, pregnancy and live birth. Therefore, the LCS could be considered as a stable and simple tool for human embryo vitrificaton.
Keywords: Clinical outcomes; Human blastocysts; Long cut straw; Survival; Vitrification.
Figures



Similar articles
-
A prospective randomized controlled trial investigating the effect of artificial shrinkage (collapse) on the implantation potential of vitrified blastocysts.Hum Reprod. 2015 Nov;30(11):2509-18. doi: 10.1093/humrep/dev218. Epub 2015 Sep 12. Hum Reprod. 2015. PMID: 26364080 Clinical Trial.
-
Associations of blastocyst features, trophectoderm biopsy and other laboratory practice with post-warming behavior and implantation.Hum Reprod. 2018 Nov 1;33(11):1992-2001. doi: 10.1093/humrep/dey291. Hum Reprod. 2018. PMID: 30265329
-
Survival of vitrified in vitro-produced bovine embryos after a one-step warming in-straw cryoprotectant dilution procedure.Theriogenology. 2015 Mar 15;83(5):881-90. doi: 10.1016/j.theriogenology.2014.11.021. Epub 2014 Nov 26. Theriogenology. 2015. PMID: 25542458
-
Pregnancy and birth outcomes following fresh or vitrified embryo transfer according to blastocyst morphology and expansion stage, and culturing strategy for delayed development.Hum Reprod. 2016 Aug;31(8):1685-95. doi: 10.1093/humrep/dew127. Epub 2016 Jun 6. Hum Reprod. 2016. PMID: 27270972
-
Outcomes of blastocysts biopsied and vitrified once versus those cryopreserved twice for euploid blastocyst transfer.Reprod Biomed Online. 2014 Jul;29(1):59-64. doi: 10.1016/j.rbmo.2014.03.001. Epub 2014 Mar 15. Reprod Biomed Online. 2014. PMID: 24794643
Cited by
-
Efficacy of embryo transfer on day 2 versus day 3 according to maternal age in patients with normal ovarian response.Clin Exp Reprod Med. 2017 Sep;44(3):141-145. doi: 10.5653/cerm.2017.44.3.141. Epub 2017 Sep 26. Clin Exp Reprod Med. 2017. PMID: 29026720 Free PMC article.
-
Effects of laser-assisted thinning versus opening on clinical outcomes according to maternal age in patients with repeated implantation failure.Lasers Med Sci. 2019 Dec;34(9):1889-1895. doi: 10.1007/s10103-019-02787-4. Epub 2019 May 1. Lasers Med Sci. 2019. PMID: 31044362
References
-
- Bielanski A, Bergeron H, Lau P, Devenish J. Microbial contamination of embryos and semen during long term banking in liquid nitrogen. Cryobiology. 2003;46:146–152. - PubMed
-
- Bielanski A, Nadin-Davis S, Sapp T, Lutze-Wallace C. Viral contamination of embryos cryopreserved in liquid nitrogen. Cryobiology. 2000;40:110–116. - PubMed
-
- Bielanski A, Vajta G. Risk of contamination of germplasm during cryopreservation and cryobanking in IVF units. Hum Reprod. 2009;24:2457–2467. - PubMed
-
- Cobo A, Kuwayama M, Pérez S, Ruiz A, Pellicer A, Remohí J. Comparison of concomitant outcome achieved with fresh and cryopreserved donor oocytes vitrified by the Cryotop method. Fertil Steril. 2008;89:1657–1664. - PubMed
-
- De Munck N, Santos-Ribeiro S, Stoop D, Van de Velde H, Verheyen G. Open versus closed oocyte vitrification in an oocyte donation programme: A prospective randomized sibling oocyte study. Hum Reprod. 2016;31:377–384. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

